Chem 336 - Spring 1999 - Organic Chemistry III
Portland State University - Dr. Carl C. Wamser
Exam 1
1. (12 points) Write a complete name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 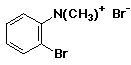
b) ![]()
c) 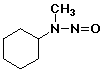
2. (15 points) Write accurate structures that illustrate each of the following:
a) the benzyne that would be formed from p-bromotoluene
b) the best resonance form of benzenediazonium ion
c) the transition state of a Claisen rearrangement (use dotted lines for bonds that are changing, and clearly indicate which ones are being broken and which ones are being made)
d) the transition state for a Diels-Alder reaction (use pictures of orbitals to indicate the bonds that are changing)
e) the most stable cation that could be formed by addition
of H+ to
1-methyl-1,3-cyclohexadiene
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) 
b) ![]()
c) 
d) 
e) 
4. (10 points) Guanine, shown below, has five different nitrogens.
However, the most basic site on guanine is not a nitrogen. Deduce
the best place to protonate guanine and explain why.
5. (10 points) The conjugate acid of morpholine has a pKa of about 8, while the conjugate acid of piperidine has a pKa of about 10.
Which amine is the stronger base? What are their approximate pKb values?
In aqueous solution at pH 9, which amine would exist mainly as an ammonium ion?
Give an explanation for the difference in basicity between these two amines.
6. (16 points) Write a complete mechanism for the reaction
of nitric acid and sulfuric acid with N,N-dimethylaniline to give
para-nitration.
Show all steps and all resonance forms for any intermediates involved.
7. (10 points) A Diels-Alder reaction can occur with compounds
other than dienes if they have a suitable arrangement of four
pi electrons, such as the case shown below.
Carefully rewrite the starting compounds to show good Lewis structures
with all the electrons. Then use electron pushing to illustrate
how they become the electrons in the final product.
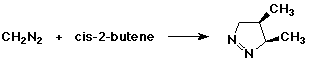
8. (12 points) Write a synthetic sequence to prepare m-bromophenol starting with benzene. Show the reagents and conditions needed for each separate step of the sequence.