Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring Term |
Quiz 22 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring Term |
Quiz 22 |
![]()
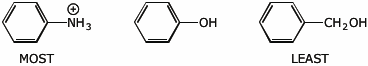
1. (1 point) Arrange the following in order with respect to their acidity.
Write MOST and LEAST under the most and least acidic compounds.
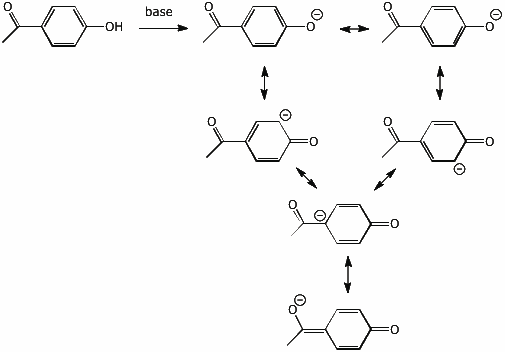
2. (2 points) Write all the resonance forms for the phenolate anion formed from the compound below.
3. (3 points) Complete the following reactions by adding the final major product.
a) 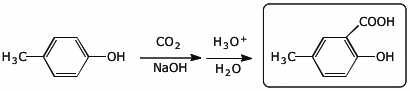
b) 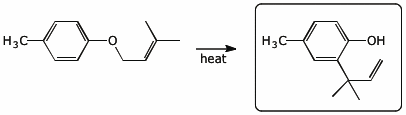
c) 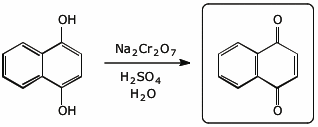
3. (2 points) Electron-withdrawing substituents (such as Cl) make phenols stronger acids. Write an acid-base reaction involving only phenol and 4-chlorophenol (and their corresponding conjugate bases) so that the reaction is favorable in the forward direction.
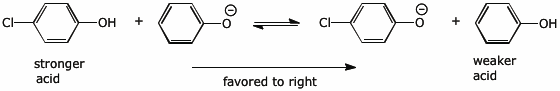
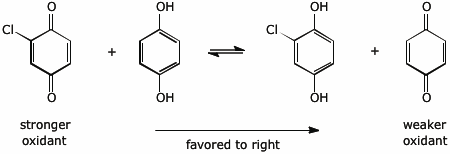
4. (2 points) Electron-withdrawing substituents (such as Cl) make quinones stronger oxidizing agents. Write a redox reaction involving only 1,4-benzoquinone and 2-chloro-1,4-benzoquinone (and their corresponding reduced forms, i.e., hydroquinones) so that the reaction is favorable in the forward direction.