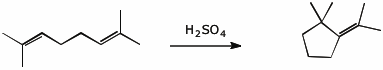Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring Term |
Exam 3 |
![]()
Organic Chemistry III |
 |
|
Professor Carl C. Wamser |
||
Chem 336 - Spring Term |
Exam 3 |
![]()
1. (20 points) Write complete structures for each of the following.
Include stereochemistry and assume pH 7.
a) cytidine-3’-monophosphate
b) BOC-Pro-Gln
c) the C-1 monoglyceride of cis-octadec-9-enoic acid with (S) stereochemistry
d) N-formyl methionine
e) deoxythymidine
2. (20 points) Complete each of the following reactions by writing the structure of the expected product, including stereochemistry.
a) ![]()
b) ![]()
c) ![]()
d) 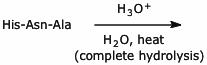
e) 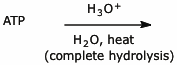
3. (15 points) Using the side chains of ten different amino acids, illustrate each of the following interactions.
Write out just the complete side chains, indicate which amino acid it is from, and show the specified interaction.
a) covalent amide bonding
b) Coulombic attraction
c) hydrogen-bonding (using uncharged side chains)
d) hydrogen-bonding (using one charged side chain)
e) hydrophobic interactions
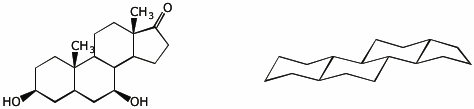
4. (10 points) Locate the substituents from the structure on the left in their proper places on the structure on the right. Circle every substituent that would be considered axial.
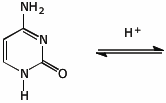
5. (15 points) Show how cytosine can be converted to its fully aromatic enol tautomer using acid catalysis. Show all resonance forms for the protonated intermediate.
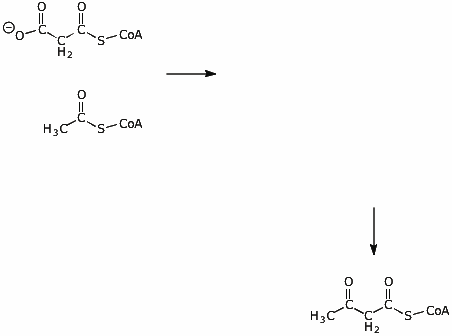
6. (10 points) Write the structure of the intermediate in the condensation reaction between acetyl CoA and malonyl CoA. Show electron-pushing arrows to show how the intermediate would be formed, and show electron-pushing arrows to show how the intermediate would be converted to the final product.
7. (10 points) Write a mechanism for the acid-catalyzed cyclization of the terpene shown below.