Chem 336 - Spring 2002
Organic Chemistry III
Dr. Carl C. Wamser

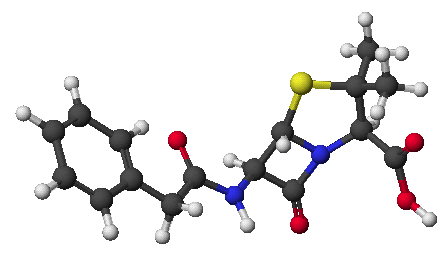
1. (15 points) Write complete names for each of the following:
a) ![]()
N,N,N-trimethyl-4-nitrophenylammonium bromide
or N,N,N-trimethyl-4-nitrophenylanilinium bromide
b) 
4-benzyl-3-methylphenol
c) 
N-nitroso-N-propyl-1-butanamine
N-nitrosobutylpropylamine
d) 
(S)-N-cyclopentyl-6-methyl-3-heptanamine
e) 
dimethyl 2-bromomalonate
dimethyl 2-bromopropanedioate
2. (15 points) Arrange the following sets of compounds in order with respect to the property indicated. Write "MOST" and "LEAST" under the compounds with the highest and lowest values of the property.
a) acidity
MIDDLE / / MOST / / LEAST
b) acidity
MIDDLE / / LEAST / / MOST
c) basicity
MOST / / MIDDLE / / LEAST
d) reactivity towards a typical nucleophile
MOST / / MIDDLE / / LEAST
e) ease of oxidation
MIDDLE / / LEAST / / MOST
3. (15 points) Complete each reaction by adding the missing part: either the starting materials, the necessary reagents and conditions, or the final major product. Include stereochemistry if it is specific.
a) 
b) 
c) 
d) 
e) 
4. (15 points) Write a complete mechanism for the mixed Claisen condensation of ethyl acetate with ethyl formate.
Show all steps and all resonance forms for any intermediates involved, and show electron-pushing arrows in each step.
Explain why the reaction requires one equivalent of base (rather than being catalytic in base), and why an acid workup is needed to recover the final product.

The final product is a stronger acid than ethanol, so it loses H+ in the basic environment and uses up one equivalent of NaOEt. The final product can be recovered by an acid workup.
5. (15 points) Write a sequence of reactions that could be used to convert benzene to m-chlorobenzyl amine. Your sequence must pass through m-chloronitrobenzene.

6. (10 points) Write the sequence of reactions that would be used in a malonic ester synthesis of octanedioic acid, using 1,4-dibromobutane.

7. (15 points) All of the following compounds hydrolyze in water easily. Predict the products expected from each.
a) 
b) 

c) ![]()
![]()
d) ![]()

e) 
