Organic Chemistry II

Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Chapter 12 Homework Answers |
![]()

2. Consider whether the following substituents would be activating or deactivating, and also predict whether they would be meta- or ortho,para-directing. Write the best Lewis structures for each substituent to justify your answers.
-CNO , -NCO , -OCN
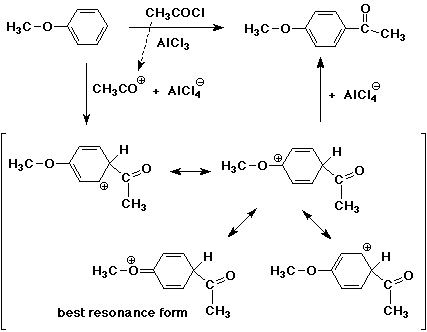
3. Write a complete mechanism for the Friedel-Crafts reaction of anisole with acetyl chloride, including all resonance forms for the intermediate. Indicate the most stable resonance form.
4. Pentachlorophenol (PCP) has been used as a wood preservative, but has the problem of generating polycyclic compounds (commonly called dioxins) that are toxic. Write a mechanism for the reaction below.

