Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 3 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 3 |
![]()
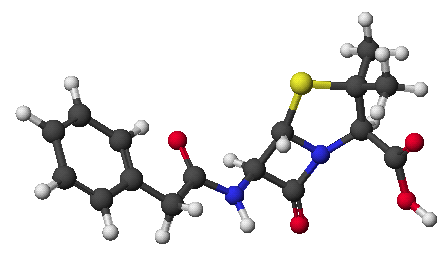
1. (15 points) Write complete names for each of the following.
a) 
b) ![]()
c) 
d) 
e) 
2. (15 pts) Write complete structures for the following.
a) benzaldehyde hydrazone
b) an epoxide that is a meso compound
c) chlorodimethylsulfonium chloride
d) 18-crown-6
e) the neutral tetrahedral intermediate in the hydrolysis of ethyl acetate
3. (15 pts) Arrange the following in order with respect to the property indicated. Write MOST under the compound with the highest value and LEAST under the compound with the lowest value.
a) acidity
![]()
b) basicity
![]()
c) water solubility
![]()
d) rate of hydration

e) acidity

4. (15 pts) Complete each of the following reactions by adding the missing part: either the starting materials, the necessary reagents and conditions, or the expected major product.
a) 
b) 
c) 
d) 
e)

5. (15 points) Using epoxide reactions, show how you would convert cyclopentene to each of the following compounds. You may use each target as the starting point for the next target, if appropriate. You do not need to create your reagents, just show the reactions that would be needed.

6. (15 points) Write a complete mechanism for the acid-catalyzed reaction shown below. Show all steps in the mechanism and all resonance forms for any intermediates. Electron-pushing arrows are optional. Use of H+ and - H+ is acceptable.

7. (10 points) Since halides are important components of so many reactions, it is important to be able to generate them from a variety of sources. Complete each of the following reactions by showing the appropriate reagent for each of the following conversions.
Every one of the following should use different combinations of reagents and conditions.
a) ![]()
b) ![]()
c) ![]()
d) ![]()
e) ![]()
f) ![]()
g) ![]()
h) ![]()
i) ![]()
j) ![]()