Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Quiz 11 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Quiz 11 |
![]()
1. (1 point) Write the structure of m-chlorobenzylbromide.

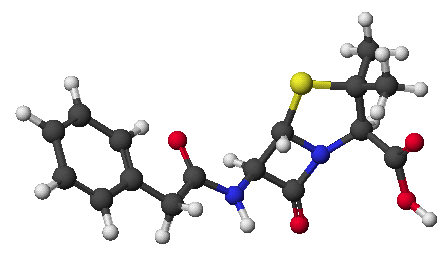
2. (1 point) Give a complete name for the compound below.

3. (1 point) Write the three resonance forms for naphthalene (C10H8).

4. (2 points) Complete each of the following reactions:
a) 
b) 
5. (5 points) The compound shown below undergoes SN1 substitution via a carbocation. Write all the resonance forms for the cation intermediate.
Explain why the meta isomer does not react as quickly as the para isomer.
