Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 3 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 3 |
![]()
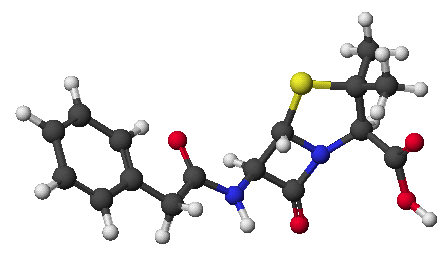
1. (15 points) Write complete names for the following compounds, including stereochemistry if it is specifically shown.
a) ![]()
b) ![]()
c) 
d) ![]()
e) 
2. (15 points) Write complete structures for the following.
a) 2,2-dibenzyloxirane
b) ammonium acetate
c) the best resonance form of protonated cyclohexanone
d) the hemiacetal from benzaldehyde and phenol
e) isobutyl mercaptan
3. (15 pts) Arrange the following in order with respect to the property indicated. Write MOST under the compound with the highest value and LEAST under the compound with the lowest value.
a) acidity
![]()
b) reactivity in nucleophilic addition reactions

c) boiling point
![]()
d) nucleophilicity
![]()
e) acidity
a) 
b) 
c) 
d) 
e) 
5. (15 points) All of the following compounds undergo ring-opening hydrolysis in dilute aqueous acid. Show the products expected in each case (complete hydrolysis).
a) 
b) 
c) ![]()
d) ![]()
e) 
6. (10 points) Show a sequence of reactions that could be used to synthesize the compound shown below. All carbons in your final product must come from alcohols having three carbons or fewer, plus any needed solvents or reagents (including NaCN and CO2).

7. (15 points) Write a complete mechanism for the acid-catalyzed reaction shown below. Show all steps in the mechanism and all resonance forms for any intermediates.
Electron-pushing arrows are optional. Use of H+ and - H+ is acceptable.
![]()