Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2009 |
Quiz 18 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2009 |
Quiz 18 |
![]()
1. (2 pts) Identify the most acidic hydrogen in the compound below, and draw all the resonance forms of the corresponding conjugate base.

2. (2 pts) Write the structure that would be obtained if all of the ketones below were in their enol form.

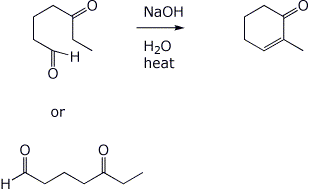
3. (2 pts) The compound below can be formed by an intramolecular aldol condensation. Show what the original compound must have been.
4. (4 pts) Write a complete mechanism for the acid-catalyzed bromination shown below. Show all steps and all resonance forms. (Hint - first form the enol.)
