Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2009 |
Exam 3 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2009 |
Exam 3 Answer Key |
![]()
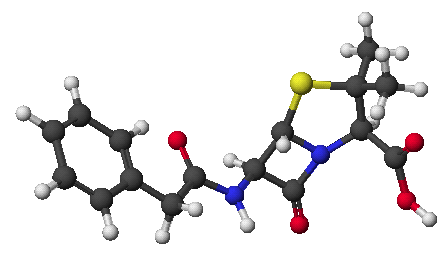
1. (15 points) Write complete names for the following compounds, including designation of stereochemistry.
a) 
b) 
c) 
2. (15 points) Write accurate structures for the following compounds:
a) (1R,2S)-1-methyl-1,2-epoxycyclohexane

b) cis-4-methylthiocyclohexanol
![]()
c) the aldol product from pentanal (no dehydration)

d) the dimethyl acetal of cis-2-butenal
![]()
e) the most stable resonance form of the enolate of 1,3-cyclohexanedione

3. (15 points) Arrange the following sets in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) acidity
![]()
b) amount of enol at equilibrium
![]()
c) amount of (R) product expected

d) basicity
![]()
e) amount of hydrate at equilibrium
![]()
4. (15 points) Complete each of the following reactions by adding the missing part:
a) 
b) 
c) 
d) 
e) 
5. (15 points) Write a complete mechanism for the complete acid-catalyzed hydrolysis of the acetal shown below. Show all steps and show all resonance forms for all intermediates.

6. (15 points) Write a complete mechanism for the base-catalyzed aldol reaction, including dehydration, of the aldehyde shown below. Show all steps and show all resonance forms for all intermediates.

7. (10 points) Starting from 2-propanol as the only source of carbon, write two different synthetic sequences to make 2,3-dimethyl-2-butene -- one using a Grignard reagent and one using a Wittig reaction.
