Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2009 |
Exam 2 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2009 |
Exam 2 Answer Key |
![]()
1. (15 points) Write complete names or clear structures for each of the following:
a) 
b) ![]()
c) ![]()
d) an example of a Cu(I) compound that follows the 18-electron rule with CN- ligands
e) a chromium compound in the +6 oxidation state
![]()
2. (20 points)
a) Identify the C5 compound with IHD = 0 that would give only a singlet in its proton NMR spectrum.
b) The mass spectrum of benzyl bromide gives, besides the expected M and M+2 peaks, strong peaks at m/z = 91 and m/z = 77. Identify the expected structure of these fragments.

c) The C-13 NMR spectrum of chlorocyclohexane has only four absorptions at 60, 37, 26, and 24 ppm. Identify which carbons give rise to each of these peaks.
d) Explain how you would use IR spectra to distinguish between the following.

3. (15 points) Complete each of the following reactions by writing the structure of the expected product:
a) 
b) 
c) 
d) & e) 
4. (15 points) In each of the reactions below, the product is a tertiary alcohol. Identify the products with clear structures.
a) 
b) 
c) 
5. (20 points) Show how to prepare each of the two compounds below using Grignard coupling reactions for the C-C bond-forming steps. All the carbons in the products must originate from benzene or alcohols having three or fewer carbon atoms. You may use any needed reagents or solvents.



6. (15 points) Identify the unknown compound based on the data below.
Molecular Formula is C9H12O .
Spectra are shown below and on the following
page.
Correlate spectral features with the structure.
H-NMR:
Areas of the
peaks (left to right) are 5 : 2 : 1 : 1 : 3 .
Aryl 5H at 7.3 ppm
CH2 adjacent to O (doublet) at 3.6 ppm
CH with multiple neighbors (benzylic) at 2.9 ppm
OH (singlet) at 1.6 ppm
CH3 with one neighbor at 1.2 ppm

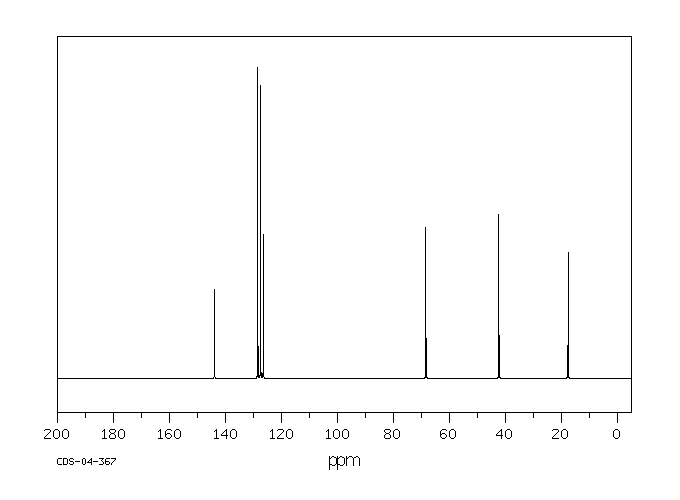
C-13 NMR:
4 different aryl C at 120-150 ppm
C next to O at 68 ppm
benzylic C at 42 ppm
CH3 at 18 ppm
IR:
strong, broad O-H stretch at 3200 - 3500 cm-1
sp2 C-H stretches between 3000 - 3100 cm-1
aryl C=C stretches around 1600 cm-1
