
Chem 335 - Winter 2002
Organic Chemistry II
Dr. Carl C. Wamser

Identify the unknown compound based on the spectral data provided.
For each item of data, indicate what structural information it provides.
Molecular Formula - C10H12O
Index of H deficiency = 5 (indicates rings + pi bonds = 5)
Mass Spectrum - parent molecular ion at 148
indicates molecular weight = 148
Infrared Spectrum - strong absorption at 1684 cm-1
indicates likely presence of a C=O bond
H-1 and C-13 spectra below (identify the absorptions for each H and C)
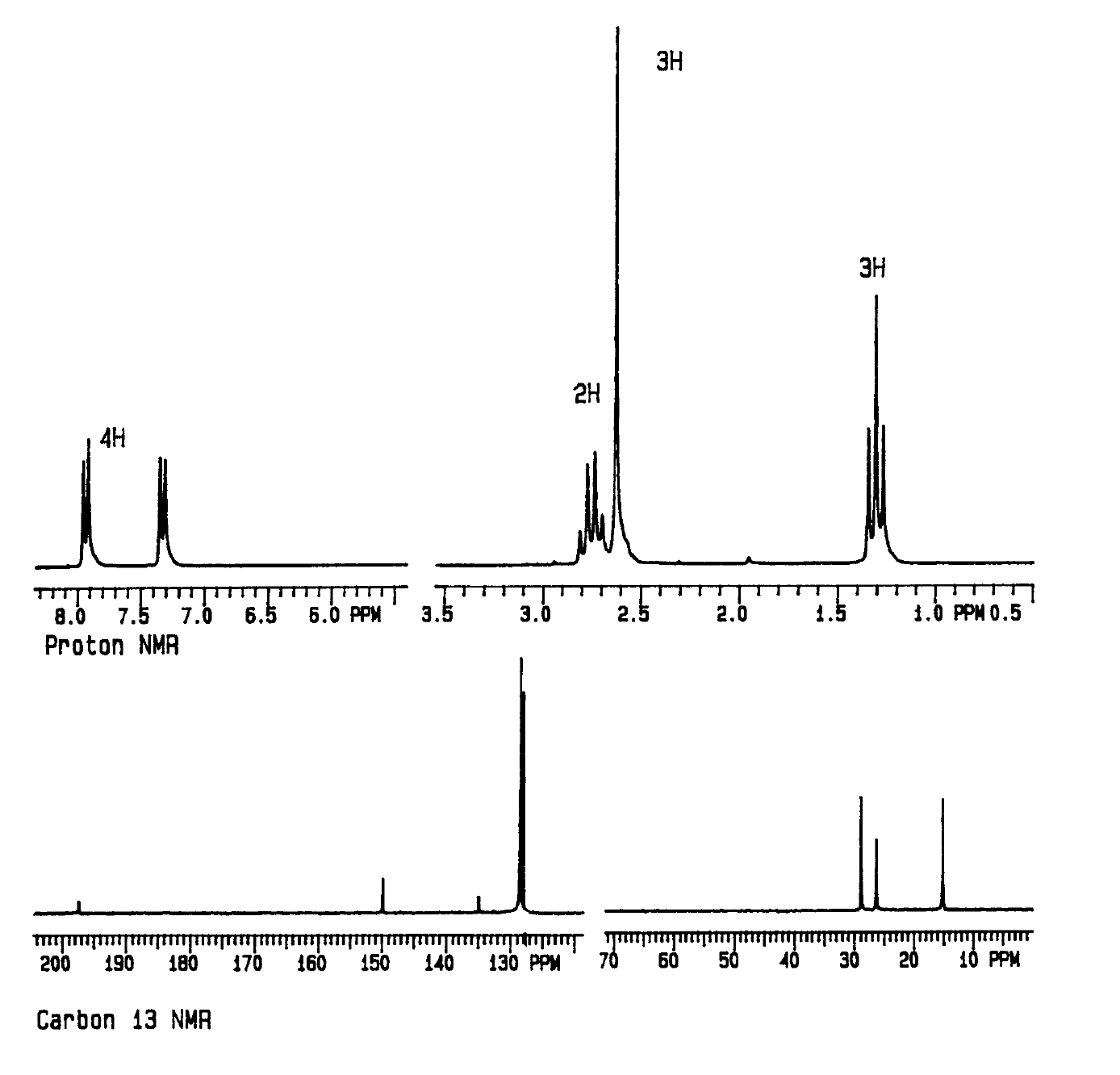
H-1 spectrum:
a - 1.3 ppm, triplet, 3H
b - 2.7 ppm, quartet, 2H
d - 7.3 or 7.9 ppm, doublet, 2H
e - 7.9 or 7.3 ppm, doublet, 2H
h - 2.6 ppm, singlet, 3H
C-13 spectrum:
a - 15 ppm
b - 26 or 29 ppm
c - 135 ppm
d - 128 ppm
e - 128 ppm
f - 149 ppm
g - 197 ppm
h - 29 or 26 ppm
Note - the spectral data are also quite consistent with the isomer that has the methyl and ethyl reversed (but the spectra are actually those of the above compound).