Chem 335 - Winter 2002
Organic Chemistry II
Dr. Carl C. Wamser

1. (8 points) Write complete names for each of the following:
a) 
b) 
2. (6 points) Write accurate illustrations for each of the following:
a) the energy level diagram for the pi molecular orbitals of 1,3,5-hexatriene
b) the orbital symmetry of the HOMO of 1,3-butadiene
3. (10 points) Write an accurate structure for the molecule below that illustrates the relative geometry of all the bonds.
Identify the hybridization of every carbon.
Identify the shortest carbon-carbon bond.
Identify the shortest carbon-hydrogen bond.
Identify the most acidic hydrogen.
4. (15 points) Complete each reaction by adding the missing part: either the starting materials, the necessary reagents and conditions, or the final major product.
a) 
b) 
c) 
d) ![]()
e) 
5. (16 points) Write a complete mechanism for the addition of one equivalent of HBr to 3,4-dimethyl-1,3-pentadiene.
Show all steps, all resonance forms, and all possible products.
6. (15 points) Write a synthetic sequence of reactions that could be used to make 1-penten-4-yne starting from acetylene and methyl iodide as the only sources of carbon. You may use any needed inorganic reagents or solvents.
(Hint - allyl bromide would be useful)
7. (15 points) Identify the unknown compound based on the spectral data below.
Letter the positions in your proposed structure and use the letters to clearly indicate assignments for all peaks in each of the NMR spectra.
Also indicate assignments for at least two absorptions in the IR spectrum.

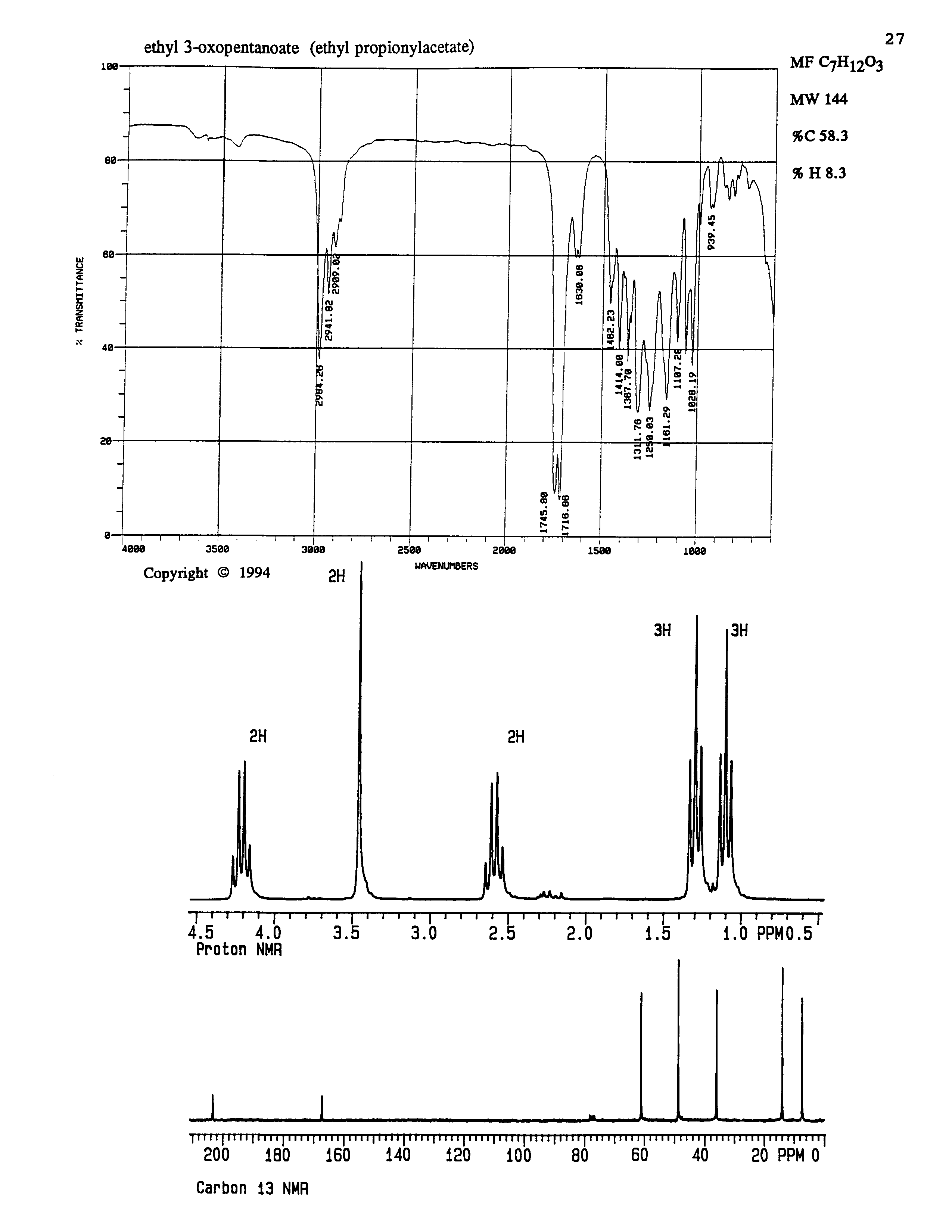
8. (15 points) Identify the unknown compound based on the spectral data below.
Letter the positions in your proposed structure and use the letters to clearly indicate assignments for all peaks in each of the NMR spectra.
Also indicate assignments for at least two absorptions in the IR spectrum.