Organic Chemistry II
Dr. Carl C. Wamser
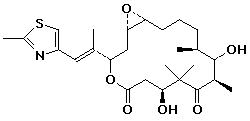 Epothilone
A
Epothilone
A
Mystery molecule #7 has been retired undefeated.
Reference to this can be found in Chemical & Engineering News, January 31, 2000, page 7.
Epothilones A and B are promising new anticancer agents, for which the genes have been recently isolated, sequenced, and expressed.
On the other hand there was one honorable mention, indicating that someone was trying hard to solve it, plus add some humor too. Thanks, Rebecca.
Hi Dr. Wamser,
Well, knowing that this is the last weekend for the weekly
emolecule to stay
on your website, I thought I'd give it one last try.... Also!
To make this
even more fun, I've given you choices in type/name/description
:) Here
are the choices....
******************************************
Choice A: gelatin methacrylamide.
Dynamic shear oscillation measurements at small strain were
used to
characterize the viscoelastic properties and related differences
in the
molecular structure of hydrogels based on gelatin methacrylamide.
Gelatin was
derivatized with methacrylamide side groups and was subsequently
cross-linked
by radical polymerization via photoinitiation. Synthesis and Characterization
of Modified Gelatin. Gelatin methacrylamide was prepared by reaction
of
gelatin with methacrylic anhydride.
http://pubs.acs.org/subscribe/journals/bomaf6/jtext.cgi?bomaf6/asap/html/bm99
0017d.html
*************************************
Choice B: selectins/sulfated sialyl LewisX (sLeX)
A highly practical synthetic approach is described for artificial
L- and
P-selectin blockers. The synthesis involves radical bi- and
terpolymerizations of p-(N-acrylamido)phenyl 3- or/and 6-sulfo--D-galactoside
with allyl -L-fucoside in the presence of acrylamide. Each of
the two
glycosyl monomers constructs a key carbohydrate module responsible
for
selectins/sulfated sialyl LewisX (sLeX) bindings. Whereas an acrylamide
copolymer carrying 3-sulfo-galactoside showed no activity for
any selectins,
the fucosylated terpolymer showed a potent activity to block both
of P- and
L-selectins/sLeX binding at a concentration of a few micrograms
per
milliliter. The enhanced activity is apparently ascribed to the
cooperative
binding effects of the fucoside and the 3-sulfo-galactoside residues.
http://pubs.acs.org/subscribe/journals/bomaf6/jtext.cgi?bomaf6/asap/html/bm990
011o.html
------------------------------------------------------------------------------
Choice C: Chitosan
Natural biodegradable polymers are an important class of materials
that can
act as a temporary scaffold for the transplanted cell before being
substituted by an extracellular matrix. Various groups have investigated
the
production of chitosan-based porous materials.31,32 Chitosan is
a partially
deacetylated derivative of chitin. Chitosan has an amino group
that permits
its dissolution in an acidic solvent (pH < 6). The high density
of amino
groups on the polymer backbone imparts on chitosan a hemostatic
characteristic.33,34 This property may cause complications by
coagulating
blood when used as scaffolds in tissue transplantation. Chitin,
the
acetylated form, has an acetyl group attached to the C2 position
instead of
the amino moiety. This greatly reduces the polycationic character
of the
polymer backbone and diminishes the hemostatic property. Ironically,
the
amide group that gives chitin the advantage over chitosan also
lessens
greatly its solubility in most organic solvents. The presence
of amide
functionality imparts a rigid three-dimensional hydrogen-bonded
molecular
structure and renders chitin as only soluble in an aprotic solvent
system
such as a dimethyl acetamide/lithium chloride mixture.
http://pubs.acs.org/isubscribe/journals/bomaf6/jtext.cgi?bomaf6/asap/html/bm00
5503b.html
*******************************************
Choice D: Water
Also known as Dihydrogen oxide; Ice; Aqua; Hydrogen oxide;
Steam; Snow. This
incredible molecule has the formula H2O and weight of about 18
g. It is a
weak acid and base and has much surface tension, heat capacity,
and average
density.
http://www.chemfinder.com/result.asp; my brain
*******************************************
Is that your final answer? :)
R.H.