Organic Chemistry II
Dr. Carl C. Wamser
Brown & Foote, pages 674 - 685 :
Problems 17.13 - 23 , 25 - 37 , 40 - 42 , 48
1. Write complete names for each of the following:
a) 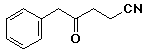
4-oxo-5-phenylpentanenitrile
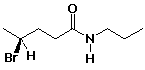
(S)-4-bromo-N-propylpentanamide
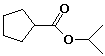
isopropyl cyclopentanecarboxylate
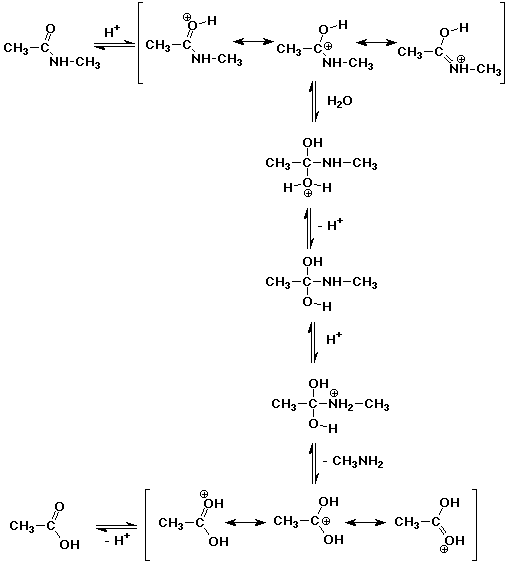
3. A typical fat has ester functional groups (most typically it
is a triester between three fatty acids and glycerol). Glycerol
is 1,2,3-propanetriol, and a fatty acid might be decanoic acid.
Write the structure for the triester made from these components.

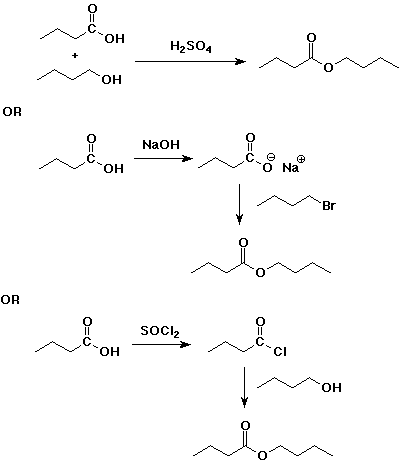
4. Using butanoic acid as the only organic starting material,
show how to prepare:
a) 1-butanol
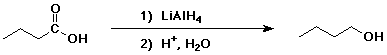
b) 1-bromobutane
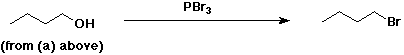
c) butyl butanoate
d) butanamide
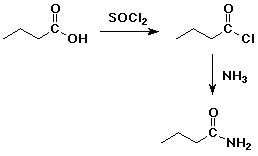
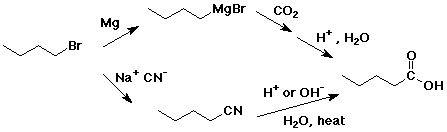
e) pentanoic acid