Organic Chemistry II
Dr. Carl C. Wamser
1. (15 points) Write a complete name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 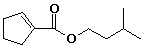
b) 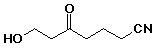
c) 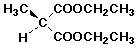
2. (15 points) Write accurate structures that illustrate each of the following:
a) lithium diisopropylamide
b) the best resonance form for the anion of malonic ester
c) a beta-lactone
d) the kinetically-controlled enolate anion of 3-methyl-2-butanone
e) N-ethyl cis-2-pentenamide
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) 
b) 
c) 
d) 
e) 
4. (10 points) Show the sequence of steps that would be used in a malonic ester synthesis of the compound shown below. Show the compounds that would be formed at each step of the synthesis, but full mechanisms are not required.
5. (10 points) Write a complete mechanism for the base-catalyzed hydrolysis of N,N-dimethylacetamide. Show all steps and all resonance forms for any intermediates involved.
6. (20 points) Write a complete mechanism for the acid-catalyzed conversion shown below. Show all steps and all resonance forms for any intermediates involved.
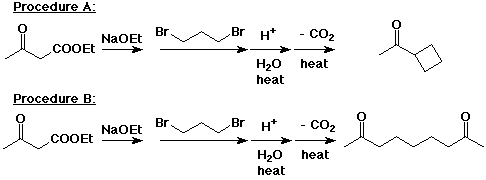
7. (15 points) Depending on the experimental conditions used, an acetoacetic ester synthesis can be used with dihalo compounds to make either a cyclic ketone or an acyclic diketone. Show the steps involved in each of the following two conversions, and describe the differences in experimental conditions that would be necessary to bring about the different results.