Organic Chemistry II
Dr. Carl C. Wamser
1. (10 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) (3 pts) 
(R)-2,3-dihydroxypropanal
b) (3 pts) 
4,4-dimethoxycyclohexanone
c) (4 pts) 
(S)-2-chloro-4-ethoxybutanal
2. (15 points)
a) illustrate the McLafferty rearrangement of 3-hexanone

b) show the products from complete hydrolysis of (CH3)2C=NCH2CH3
![]()
c) show the products from complete hydrolysis of ![]()

d) identify the compound of formula C5H12 that shows only one H-1 NMR peak
2,2-dimethylpropane (neopentane)
e) identify the compound of formula C3H6 that shows no UV absorption
cyclopropane
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) 
b) 
c) 
d) 
e) 
4. (10 points) Write a complete mechanism for the acid-catalyzed bromination shown below. Show all steps and all resonance forms for any intermediates involved.
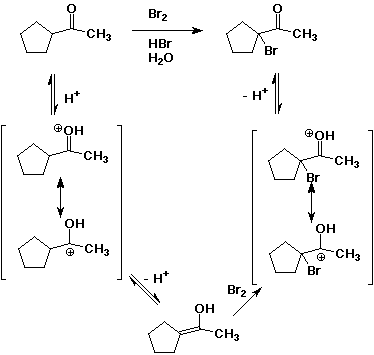
5. (10 points) Write a complete mechanism for the formation of a ketal under the conditions shown. Show all steps and all resonance forms for any intermediates involved.

6. (15 points) Identify the unknown compound from its spectral
data.
Specifically assign the H-1 NMR absorptions by letter designation.
Molecular formula: C7H14O
Infrared: no significant absorptions > 3000 cm-1 , strong absorption at 1735 cm-1
C-13 NMR: 190 ppm , plus 5 different peaks between 20 - 60 ppm
H-1 NMR:
a) 9.7 ppm, 1H, triplet
b) 2.5 ppm, 2H, doublet
c) 1.3 ppm, 2H, quartet
d) 1.0 ppm, 6H, singlet
e) 0.9 ppm, 3H, triplet
7. (15 points) There are three carbonyl compounds that could have the molecular formula C4H8O. Write structures and IUPAC names for all three, and describe their expected H-1 NMR spectra (use the format of the previous question to describe the spectral data).
a) a ketone
butanone CH3CH2COCH3
2.1-2.3 ppm, 3H, singlet
2.2-2.6 ppm, 2H, quartet
0.8-1.0 ppm, 3H, triplet
b) an aldehyde
butanal CH3CH2CH2CH=O
9.5-10.1 ppm, 1H, triplet
2.2-2.6 ppm, 2H, multiplet
1.2-1.4 ppm, 2H, multiplet
0.8-1.0 ppm, 3H, triplet
c) another aldehyde
2-methylpropanal (CH3)2CHCH=O
9.5-10.1 ppm, 1H, doublet
2.2-2.6 ppm, 1H, multiplet
0.8-1.0 ppm, 6H, doublet
8. (10 points) Show two different ways in which the following synthetic conversion could be accomplished. Use 1-bromobutane for the additional carbons.

a) using a Grignard reagent

b) using a Wittig reagent
