Organic Chemistry IProfessor
Carl C. Wamser |
|
 |
![]()
Exam 1 Answer Key
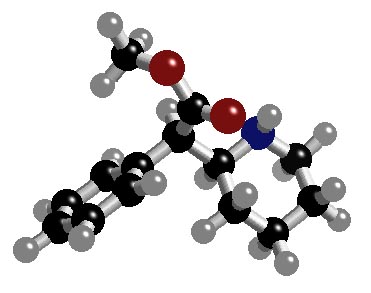
1. (15 points) Write complete names for each of the following.
a) ![]()
3-bromo-1,1,1-trifluoro-5,5-dimethylhexane
b) 
2-bromo-2-cyclopentyl-5-iodo-6-methylheptane
c) 
trans-4-isobutylcyclohexanol
trans-4-(2-methylpropyl)cyclohxanol
d) 
5-methylbicyclo[2.2.2]octan-2-ol
e) 
1-chloro-5-cyclobutyl-3-methyl-2-pentanol
2. (15 points) Write accurate structures for the following:
a) sec-butyl free radical
![]()
b) the best conformation of cis-1,3-dimethylcyclohexane

c) a 3° alcohol of formula C5H12O

d) 2-methylspiro[3.5]nonane
![]()
e) the early transition state for the reaction of CH4 with F•

3. (15 points) Arrange each of the following in order with respect to the property indicated. Write “MOST” under the compound with the highest value and “LEAST” under the compound with the lowest value.
a) stability

b) boiling point

c) dipole moment

d) reactivity towards HBr

e) amount of equatorial methyl at equilibrium

4. (10 points) Complete the following acid-base reactions and cite specific pKa data to determine whether the reaction will be favored to the right or to the left.
a)

b)

c)

Of all the compounds on this page (including the ones you wrote), indicate which one is the strongest base.
![]()
5. (15 points) Write all three staggered conformations of 2-iodo-3-methylbutane, using Newman diagrams viewing down the C2-C3 bond.
Assuming iodine is larger than a methyl, predict the relative stabilities of the three conformations.

6. (15 points) Using the table of bond dissociation energies, calculate delta H for each of the following reactions.
Cite the specific BDE values that you use. Use units of kcal/mol.
a)

b)

c)

7. (15 points) Write a complete mechanism for the reaction of cyclopentanol with HBr. Show all steps and show electron-pushing arrows for each step.

Write an approximate potential energy diagram for this reaction, showing all steps and particularly identifying the rate-determining step.