Chem 334 - Summer 1998 - Organic Chemistry I
1. (15 pts) Write complete IUPAC names for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 
b) 
c) 
2. Complete the following structures by adding the missing substituents in their appropriate places:
a) (5 pts) 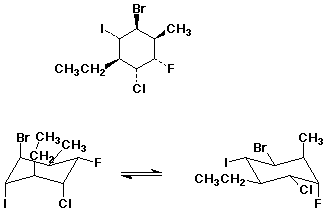
b) (4 pts) 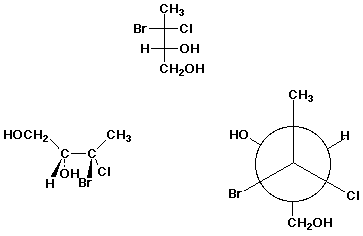
3a. (10 pts) Write the structure of (R)-1-bromo-3-chloropentane.
Write the structure of the product that would be expected from the reaction of this compound with just one equivalent of NaCN in DMSO.
Include a clear indication of its stereochemistry and its (R) or (S) designation.
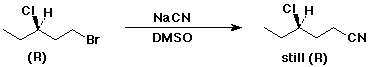
3b. (6 pts) Naproxen, the active ingredient in Aleve and other anti-inflammatory drugs, is synthesized by a process that gives the major isomer, shown below, in 97% enantiomeric excess.
Give the (R) or (S) designation to the stereoisomer shown above.
(S)
What would be the optical purity of a sample of the synthetic material cited above?
97%
What would be the percentage composition of each of the two enantiomers in the synthetic material?
98.5% (S) and 1.5% (R)
4. (10 pts) Write both chair conformations for cis-1-chloro-4-ethylcyclohexane.
Do the same for the trans isomer.
Use the data in the attached table to calculate delta G between the two chair conformations for each compound.


5. (10 pts) There are five constitutional isomers of formula C3H6BrCl .
Write either structures or names (or both if it is helpful) for each of the isomers.
Indicate possible stereochemistry by designating each one as either chiral or achiral. For each chiral isomer, indicate each of the stereocenters and identify how many stereoisomers would be expected.
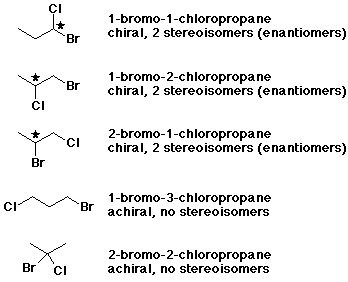
6a. (5 pts) For the reaction indicated below, write the product and describe its expected stereochemistry.

the product is still a cis compound
b) (4pts) Write the expected rate law for the reaction (represent the alkyl bromide as RBr).
Rate = k [ RBr ] [ CH3O- ]
c) (3 pts) Predict how the observed rate of the reaction would change if the concentration of both reactants were doubled.
the rate should be 4x faster
d) (3 pts) Predict how the observed rate of the reaction would change if the solution were diluted to twice its original volume with additional methanol.
the rate should be 4x slower
(the concentration of each reactant would be halved)
7. (10 pts) Write a complete mechanism that explains the following conversion. Show all steps.
8. (15 pts) The reactions below proceed by SN2 mechanisms.

Write structures for compounds A, B, and C. Indicate why A, B, and C are chiral molecules by identifying the stereocenters in each compound.
Explain why the products from these reactions are expected to be racemic.
From the achiral compound X, there will be equal amounts of the two enantiomers of A, since there should be no preference for bonding to either side of the ring.
Explain why the expected amounts of B and C are not necessarily equal.
B and C are diastereomers of one another - one will have the two methyl groups cis and the other will have the two methyl groups trans. During the bonding in the SN2 mechanism, the approaching methyl will detect a difference between the two sides of the ring.