Chem 334 - Summer 1998 - Organic Chemistry I
1. (15 pts) Write complete IUPAC names for each of the following compounds:
a) ![]()
b) 
c) 
2. (15 pts) Write accurate structures for each of the following:
a) a Newman diagram of sec-butyl bromide
b) the best Lewis structure for HCONH2
c) the gauche conformation of 1-bromopropane
d) the most stable free radical that has the formula C5H11
e) an amine that includes a tert-butyl group
plus other possibilities
3. (15 pts) For the molecule shown below:
a) What is its molecular formula?
C8 H12 O2
b) Identify the hybridization of each carbon atom on the picture below.
c) Identify each carbon as primary, secondary, or tertiary on the picture below.
d) Identify three functional groups (circle them and name the family).
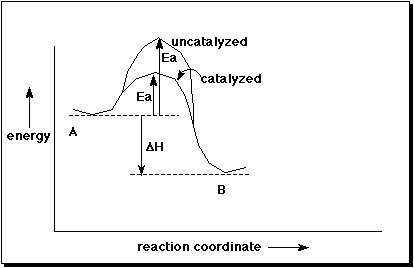
4. (10 pts) Consider the general reaction of A to B. If a catalyst is added, describe how delta H and Ea would be expected to change.
Draw a simple potential energy diagram that illustrates a catalyzed and uncatalyzed reaction of A to B.
5. (15 pts) Calculate delta H for each of the following reactions, using the data from the attached tables. If you can't find the exact structure of interest, use an appropriate analog.
a) ![]()
delta H = 84 - 110 = - 26 kcal/mole
b) ![]()
delta H = 93 + 46 - 67 - 87 = - 15 kcal/mole
c) ![]()
delta H = 77 + 119 - 92 - 107 = - 3 kcal/mole
6. (10 pts) Write all the staggered conformations for 2-bromo-2-methylbutane, looking down the C2-C3 bond. Identify the lowest energy and the highest energy of the three structures. (Bromine is bigger than a methyl)

7. (10 pts) Identify by name and structure all of the possible monochlorination products from reaction of chlorine with 3-methylpentane. Using the selectivity factors provided, calculate the expected ratio of the products.

8. (10 pts) Write a free radical chain mechanism that could accomplish the following overall reaction. Identify the likely initiation, propagation, and termination steps.
