Organic Chemistry I
Dr. Carl C. Wamser
(2 points each)
1. Give names and structures for all of the alcohol isomers, including stereoisomers (but not conformational isomers) with the formula C5H12O.
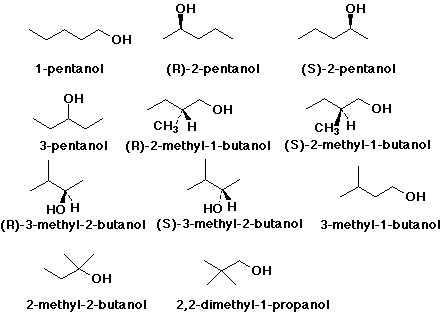
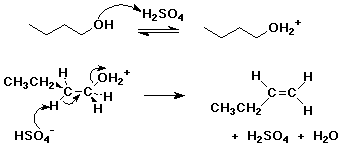
2. Write a complete mechanism for the E2 dehydration of 1-butanol by H2SO4, showing electron-pushing arrows for each step.
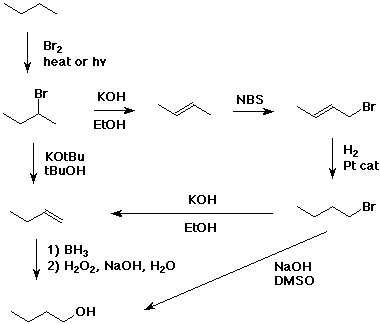
3. Write out a sequence of reactions that could be used to make 1-butanol from butane. Use the minimum number of separate steps, and only use reactions which give the desired product as the major product.
Several possibilities, including:
4. Balance the oxidation reaction in which cyclohexanol is oxidized by CrO3 (the final form of Cr is the +3 state).
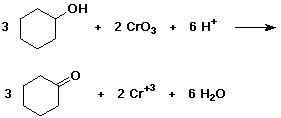
5. Write a complete mechanism that explains how (R)-2-hexanol could be converted to (S)-2-iodohexane. Indicate another likely product.
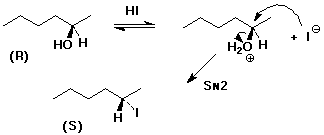
Given that this is a 2° alcohol and SN2 will be somewhat slow, elimination is also possible to give 2-hexene.
Another possibility is SN1, which would give both (R) and (S)-2-iodohexane.