Organic Chemistry I
Dr. Carl C. Wamser
1. (2 points each) Give a complete IUPAC name for each compound below, including designation of stereochemistry, including designation of stereochemistry if it is specifically shown.
a) 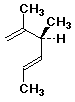
(3S,4E)-2,3-dimethyl-1,4-hexadiene
b) 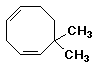
6,6-dimethyl-1,4-cyclooctadiene
2. (2 points) Identify
the isoprene units within the compound below. Clearly encircle
the carbons of each isoprene unit and indicate the "head"
and "tail" ends of each unit.
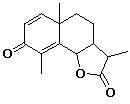
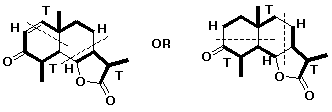
3. (4 points) Describe the difference between structure A and structure B using appropriate terminology. Predict which would be more stable.
The two structures are conformations of 1,3-butadiene, which differ only by rotation around the C2-C3 single bond.
The trans-like conformation should be more stable.
(called s-trans, where s- means a single bond conformation)