Organic Chemistry I
Dr. Carl C. Wamser
1. (4 points) The table of pKa values shows that both bicarbonate and ammonia can act as either an acid or base. Complete the following acid-base reaction of these two compounds in two different ways, and predict whether either is a favorable reaction.
![]()
![]()
Neither reaction would proceed to the right.
2. (2 points) Arrange the following in order of acidity. Indicate MOST under the most acidic and LEAST under the least acidic.
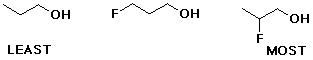
3. (4 points) Predict the major form (conjugate acid or conjugate base) of each of the following at pH 3, 7, and 11:
a) CH3COOH
CH3COOH at pH 3
CH3COO- at pH 7 & 11
b) H2O
H2O is the major form at pH 3, 7, and 11
also acceptable:
more of the conjugate acid - H3O+ - at pH 3
more of the conjugate base - HO- - at pH 11
equal amounts of the conjugate acid and base at pH 7