Organic Chemistry I
Dr. Carl C. Wamser
1. (10 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) ![]()
b) 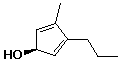
2. (15 points) Write accurate structures that illustrate each of the following:
a) perfluoroethanol
b) (R)-2-butyl tosylate
c) an E2 transition state
d) a 3° allylic alcohol
e) periodic acid
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) 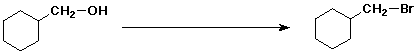
b) 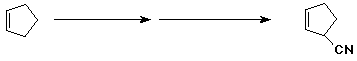
c) ![]()
d) 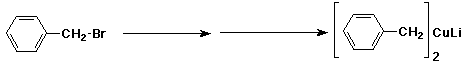
e) 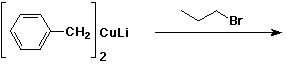
4. (15 points) There are six different products (not counting
stereoisomers) that result from free radical chlorination of 2,3-dimethylpentane.
Write the starting compound and all six product structures (again
ignore stereochemistry).
Given a selectivity of chlorine for H abstraction of 3° >
2° > 1° of 5 : 4 : 1 , circle the product that would
be expected in the greatest abundance.
5. (10 points) Use the table of bond dissociation energies to calculate DH for the following reactions:
a) (4 pts) ![]()
b) (4 pts) ![]()
c) (2 pts) Reaction (b) would be expected to follow an SN2 mechanism. How would your calculated H be different if the same reaction followed an SN1 mechanism?
6. (15 points) Write a complete mechanism for the SN1 reaction of cis-2-methylcyclohexanol with
concentrated aqueous HBr.
Show all steps and show electron-pushing arrows.
Indicate the expected major product and at least one isomeric
product that might also be expected. Indicate at least one other
product (not an isomer of the major product) that might also be
expected.
7. (20 points) The reaction shown below gives mixtures
of four different products, depending on the conditions. For
each of the four reaction conditions described below, select the
expected major product (all four are different).
Also give a brief explanation of why those conditions favor that
product.
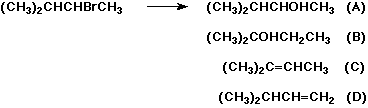
a) H2O
b) KOH in H2O
c) KOH in DMSO
d) KOtBu in tBuOH