Organic Chemistry I
Dr. Carl C. Wamser
1. (15 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) ![]()
(Z)-2-propyl-1,5-heptadiene
b) 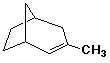
3-methylbicyclo[3.2.1]oct-2-ene
c) 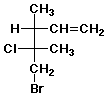
(3R,4R)-5-bromo-4-chloro-3,4-dimethyl-1-pentene
2. (15 points) Identify each stereocenter in each of the following compounds as either R or S. Identify double bonds as E or Z where appropriate. Identify the relationship, using appropriate terminology, between the two compounds in each part.
a) 
b) 
c) 
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) 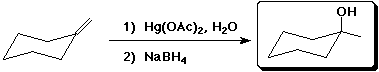
b) 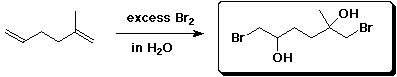
c) 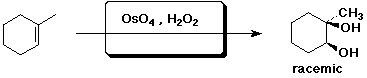
d) 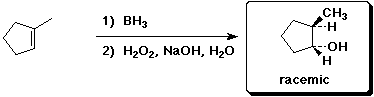
e) 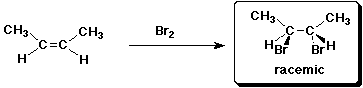
4. (15 points) Write a complete mechanism for the following reaction. Show all steps and all intermediates involved. Use electron-pushing arrows to indicate electron flow for each step. Indicate the expected stereochemistry of the product.
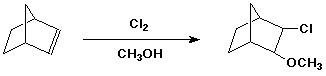
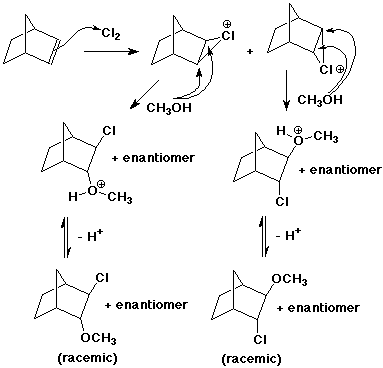
5. (15 points) You are attempting to duplicate a natural hydration reaction, which takes the E-alkene shown below to the R-stereoisomer.
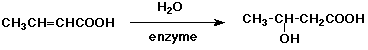
Your first attempt gives the desired product in 80% yield with 80% optical purity. The other 20% of product is the regioisomer, with 50% optical purity.
Write accurate structures that show the proper stereochemistry for:
a) the original alkene 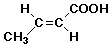
b) the desired product 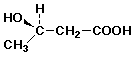
c) its enantiomer 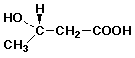
d) the regioisomer 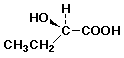
e) its enantiomer 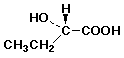
Given the indicated product yields and optical purities, identify the amounts of all of the above compounds as a percentage of total product.
80% of the total is (b + c), with an 80% enantiomeric excess (optical purity), i.e., 90% of the 80% is (b) and 10% is (c): 72% (b) and 8% (c).
20% of the total is (d + e), with a 50% enantiomeric excess, i.e., 75% is one enantiomer and 25% is the other: 15% of one and 5% of the other.
Since the yield is claimed to be 100%, presumably there is no (a) remaining.
6. (15 points) There are four alkenes of formula C4H8 , labeled below as A, B, C, and D.
There are four alcohols of formula C4H10O , labeled below as W, X, Y, and Z.
For simple acid-catalyzed hydration reactions, predict which alcohols
would be formed as the major product from each of the alkenes.
If any of the four alcohols are not formed as a product in acid-catalyzed hydration, give specific reaction conditions that you might use to make those alcohols from one of the alkenes.
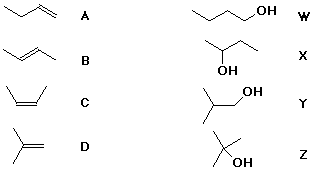
A, B, and C will all give X on hydration.
D will give Z on hydration.
To prepare W and Y, anti-Markovnikov addition will be needed, which can be accomplished with hydroboration followed by oxidation.
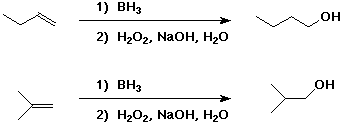
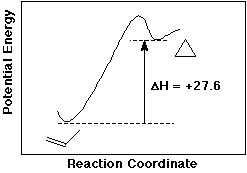
7. (10 points) Cyclopropane can be hydrogenated to propane, and the observed heat of hydrogenation is -57.7 kcal/mole
Propene is hydrogenated to propane with a heat of hydrogenation of -30.1 kcal/mole.
Indicate what you can conclude about relative stabilities, and give a plausible explanation for the difference in stabilities.
Propene is more stable than cyclopropane by 27.6 kcal/mole. This is due to the small ring, which causes serious angle strain in cyclopropane.
If propene could be isomerized to cyclopropane, would this be an exothermic or endothermic reaction? By how much?
Propene to cyclopropane would be endothermic by 27.6 kcal/mole.
Draw an approximate potential energy diagram, clearly labeling the axes, the reactant, the product, and delta H.