Organic Chemistry I
Dr. Carl C. Wamser
Nucleophilic Substitution
Recognizing Nucleophiles
Solvents
The SN2 Mechanism
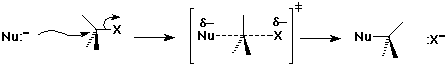
SN2 Mechanism - Evidence
SN2 Mechanism - Evidence

SN2 Mechanism - R Groups
![]()
SN2 Mechanism - X Groups
SN2 Mechanism - Nucleophiles
Solvent Effects on Nucleophilicity
SN1 Mechanism
SN1 Mechanism - Evidence
SN1 Mechanism - Evidence
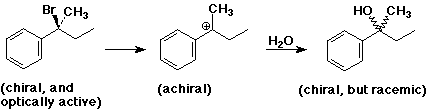
SN1 Mechanism - R Groups
SN1 Mechanism - X Groups
Solvolysis Reactions
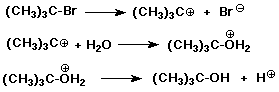
Neighboring Group Effects - Mustard Gases
Phase-Transfer Catalysis
Beta-Elimination Reactions
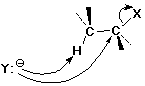
E2 Elimination Mechanism
E2 Stereochemistry
Zaitsev Rule
E1 Elimination Mechanism
Substitution or Elimination ?
Nucleophile or Base?
Reactivity Patterns
|
SN2 |
SN1 |
|
|
Reaction |
RX + Nu --> RNu + X |
same |
|
Mechanism |
concerted |
two steps |
|
Intermediate |
none |
carbocation |
|
Kinetics |
second-order |
first order |
|
Stereochemistry |
complete inversion |
nonspecific |
|
Nucleophile |
important |
unimportant |
|
Leaving Group |
important |
important |
|
Alkyl Group |
CH3 > 1° > 2° > 3°
|
3° > 2° > 1° > CH3
|
|
Occurrence |
CH3 , 1° , some 2° |
3° , some 2° |
|
Solvent Effects |
variable |
polar, protic |
|
E2 |
E1 |
|
|
Reaction |
RX + base --> C=C |
same |
|
Mechanism |
concerted |
two steps |
|
Intermediate |
none |
carbocation |
|
Kinetics |
second-order |
first order |
|
Stereochemistry |
anti periplanar |
nonspecific |
|
Base |
important |
unimportant |
|
Leaving Group |
important |
important |
|
Alkene Produced |
Zaitsev Rule |
same |
|
SN1 |
SN2 |
E1 |
E2 |
|
|
CH3X |
No |
good nucl. |
No |
No |
|
1° ( RCH2X ) |
No |
good nucl., |
No |
strong base, |
|
2° (R2CHX ) |
No |
good nucl., |
No |
strong base |
|
3° ( R3CX ) |
good nucl., |
No |
polar solvent, |
strong base |
|
Good nucl., |
Good nucl., |
Poor nucl., |
Poor nucl., |
|
|
CH3X |
SN2 |
SN2 |
SN2 |
No reaction |
|
1° ( RCH2X ) |
SN2 |
SN2 |
E2 |
No reaction |
|
2° (R2CHX ) |
E2 |
SN2 |
E2 |
No reaction |
|
3° ( R3CX ) |
E2 |
SN1 |
E2 |
SN1 |