Organic Chemistry I
Dr. Carl C. Wamser
Stereochemistry
Enantiomers
Enantiomer Examples
![]()


![]()
Chirality
Stereogenic Centers
Identifying Chiral Molecules


Properties of Enantiomers
Optical Activity
Specific Rotation
Absolute Configuration
R and S Designations
Right- and Left-Hand Views
Drawing 3-D Structures
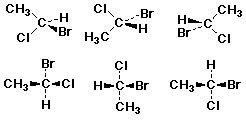
Fischer Projections
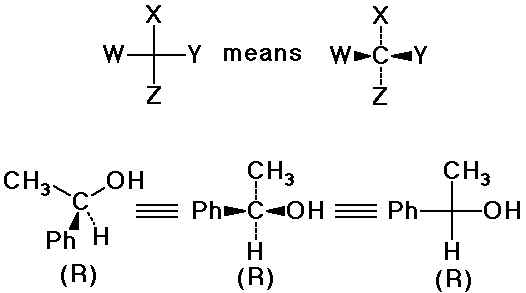
Multiple Stereogenic Centers
Diastereomers
Meso Compounds

Identifying Meso Compounds

Cyclohexane Derivatives
Configurations and Conformations of Disubstituted Cyclohexanes
|
substitution |
cis |
trans |
|
1,2-X2 |
eq,ax <==>
ax,eq |
eq,eq <==>
ax,ax |
|
1,2-XY |
eq,ax
<==> ax,eq |
eq,eq
<==> ax,ax |
|
1,3-X2 |
eq,eq <==>
ax,ax |
eq,ax <==>
ax,eq |
|
1,3-XY |
eq,eq
<==> ax,ax |
eq,ax
<==> ax,eq |
|
1,4-X2 |
eq,ax
<==> ax,eq |
eq,eq
<==> ax,ax |
|
1,4-XY |
eq,ax <==>
ax,eq |
eq,eq <==>
ax,ax |
Racemic Mixtures
Optical Purity / Enantiomeric Excess
Optical Resolution
Isomerism - Summary
Stereoisomers - Summary