1. (2 points each) Write a complete IUPAC name for each
of the following compounds:
a) 
b) 
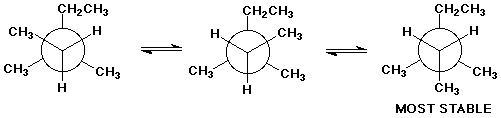
2. (3 points) Write the Newman diagrams for the three staggered conformations of 3,3-dimethylpentane, specifically looking down the C2-C3 bond. Select the most stable conformation.
3. (3 points) Write both chair conformations for trans-1-chloro-3-methylcyclohexane. Using the data table provided (textbook Table 2.4, page 77), predict the most stable and calculate delta G for the equilibrium.