1. (2 points) For each reaction below, identify the acid and the base on the left side of the equilibrium.
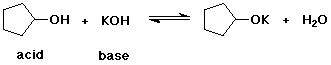
![]()
2. (2 points) Write both resonance forms for the conjugate base of a typical carboxylic acid, RCOOH. Show all lone pairs and formal charges.
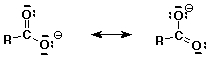
3. (1 point) An unknown acid has a pKa of 7.
That means it has a Ka value of 1 x 10^-7 .
It would be considered a ( strong / weak ) acid. (circle one)