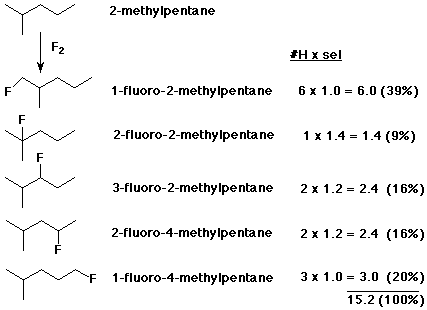Brown & Foote, pages 268 - 272:
Problems 7.8 - 19, 22, 23, 28 - 32
1. Hydrogen reacts with chlorine in a free radical chain mechanism to form HCl. Write out a complete mechanism, indicating the initiation, propagation, and termination steps. Calculate delta H for each of the steps and for the overall reaction.
overall reaction: H2 + Cl2 -----> 2 HCl / / delta H = -43 kcal/mol
initiation: Cl2 ---(heat or light)---> 2 Cl / / delta H = +59 kcal/mol
propagation (1): Cl + H2 -----> HCl + H / / delta H = +1 kcal/mol
propagation (2): H + Cl2 -----> HCl + Cl / / delta H = -44 kcal/mol
termination: H + Cl -----> HCl / / delta H = -103 kcal/mol
or Cl + Cl -----> Cl2 / / delta H = -59 kcal/mol
or H + H -----> H2 / / delta H = -104 kcal/mol
2. Fluorine is so reactive with alkanes that the rate is explosive and the products show almost no selectivity. Predict the monofluorination products expected from 2-methylpentane. Give their structures, correct IUPAC names, and the expected proportions of each, based on a selectivity of 1.4 : 1.2 : 1.0 for 3° > 2° > 1° .