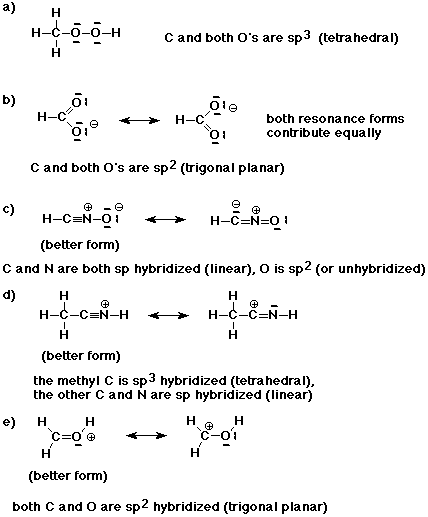Brown & Foote, pages 41 - 48 :
Problems 1.17, 20, 22, 24, 26 - 29, 32 - 39, 41 - 43, 45, 49 -
51, 53, 56 - 60
Answers to all problems from the textbook can be found in the Study Guide.
1. Write good Lewis structures for the compounds listed below. The structures are written in the standard abbreviated forms, which give some indication about the order in which atoms are bonded.
Indicate the hybridization of each atom and describe the expected geometry. If there are additional reasonable resonance forms, show them and indicate whether they contribute equally, or if not, which is the preferred resonance form.
a) CH3OOH
b) HCOO-
c) HCNO
d) CH3CNH+
e) CH2OH+