1. (15 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 
b) 
c) 
2. (15 points) Complete each of the following structures by adding the missing components. In each case, the structure on the left is complete and the structure on the right should be made into the equivalent structure from a different viewpoint.
a) 
b) 
c) 
d) 
e) Describe the relationship between the two compounds shown below, using the most appropriate terminology.
 diastereomers
diastereomers3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) 
b) 
c) 
d) 
e) 
4. (15 points ) Write a complete mechanism for the following reaction. Show all steps and all intermediates involved. Use electron-pushing arrows to indicate electron flow for each step.
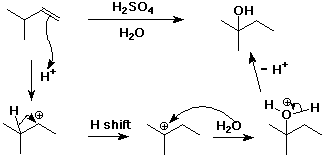
5. (15 points) Acid catalyzes the reaction shown below. Write a stepwise mechanism that illustrates how this reaction occurs, including electron-pushing arrows.

Explain how you might generate the reaction shown below.

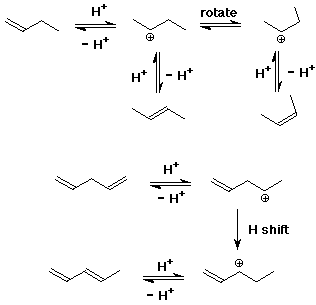
6. (15 points) Refer to the data in Table 6.3 to compare the stabilities of the following isomers. Underline the more stable isomer and calculate the difference in stability in kJ/mol.
a) 1-butene or cis-2-butene (more stable by 7 kJ/mol, or 1.7 kcal/mol)
b) cis-2-butene or trans-2-butene (more stable by 5 kJ/mol, or 1.0 kcal/mol)
c) trans-1,3-pentadiene (more stable by 28 kJ/mol, or 6.7 kcal/mol) or 1,4-pentadiene
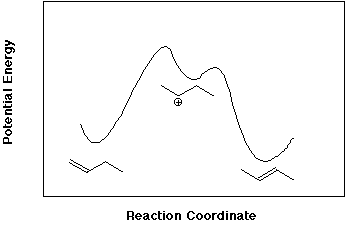
d) In fact, each of the less stable isomers can be converted into the more stable isomer by treatment with acid. Select any one of the three cases above, and write a mechanism and a potential energy diagram that illustrates reversible protonation as a means of interconversion of the two isomers.
7. (10 points) There are several different methods by which an alkene can be converted to an alcohol, some of which are listed below. For each case below, select the most appropriate method, write the specific reagents and conditions that would be used, and explain why that method would be superior to the others.
1) acid-catalyzed hydration
2) hydroboration / oxidation
3) oxymercuration / reduction
a) 
b) ![]()