Organic Chemistry I |
Exam 3 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Exam 3 |
Professor Carl C. Wamser |
![]()
1. (20 points) Write complete names for each of the following.
a) 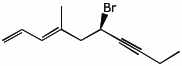
b) 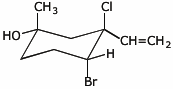
c) 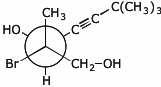
d) 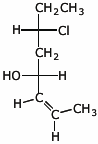
2. (15 points) Write accurate structures for the following:
a) a Newman projection of the most stable conformation of meso-2,3-dibromobutane
b) a Fischer projection of (R,R)-2,3-dibromobutane (show all carbons on the vertical axis)
c) a chair conformation of the E2 transition state as OH- reacts with
trans-1-bromo-4-methylcyclohexane
d) the most stable chair conformation of (S,S)-1,2-dimethylcyclohexane
e) (3Z,5E)-deca-3,5-dien-7-yne
3. (15 points) Arrange the following in order with respect to the property indicated. Write “MOST” under the compound with the highest value and “LEAST” under the compound with the lowest value.
a) nucleophilicity
b) ratio of substitution over elimination using KOH in ethanol
c) acidity
d) rate of hydrolysis
e) number of possible stereoisomers
4. (18 points) Complete each of the following reactions by adding the missing part: either the original reactant, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) ![]()
b) 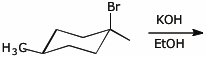
c) 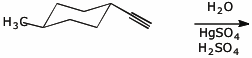
d) ![]()
e) ![]()
f) ![]()
5. (12 points) 2-Butyne can be converted to 2,3-dibromobutane in at least two different ways, as illustrated below.
Give complete names and structures for the compounds labeled as A, B, C, and D, including stereochemistry designations.
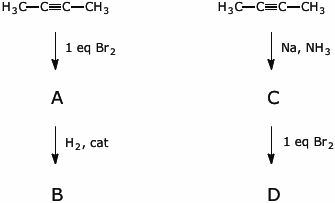
6. (20 points) Show a sequence of reactions that could be used to accomplish syntheses of the following compounds. As the starting materials, you may use acetylene and any alcohols with three or fewer carbons as the source of all the carbons in your final product. You may use any needed solvents or reagents.
a) ![]()
b) 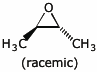
![]()