Organic Chemistry I |
Exam 2 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Exam 2 |
Professor Carl C. Wamser |
![]()
1. (20 points) Write complete names for each of the following.
a) ![]()
b) 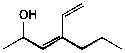
c) 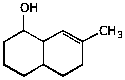
d) 
2. (15 points) Write accurate structures for the following:
a) the conjugate base of allyl alcohol
b) the most stable alkene of molecular formula C5H10
c) the epoxide of cis-2-pentene
d) a propagation step in the free radical chlorination of 2,2-dimethylpropane
e) the repeat unit of poly(vinyl chloride)
3. (15 points) Complete each of the following reactions by adding the missing part: either the original reactant, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 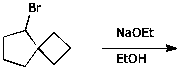
b) 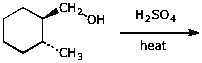
c) 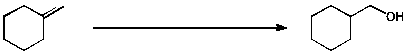
d) 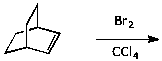
e) 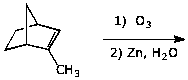
4. (15 points) Show structures for all the products expected from monochlorination of
2,2,4-trimethylpentane. Calculate the expected amounts of each, using the reactivity ratio of
3° > 2° > 1° by 5 : 4: 1. Just give relative amounts, percentages are not necessary.
5. (15 points) Use the table of bond dissociation energies to calculate delta H for the reaction below. Use units of kJ/mol.
Clearly indicate the bonds broken and the bonds made.
Write the most likely initiation step and a pair of propagation steps that would result in the overall reaction above. Calculate delta H for each of these steps.
The actual reaction gives different products. Write a balanced reaction showing those products.
6. (10 points) Write a complete mechanism for the reaction shown below. Show all steps and use electron-pushing arrows in each step.
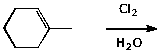
7. (10 points) Show a sequence of reactions that could be used to accomplish the following conversions.
a) 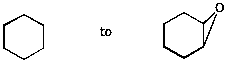
b) 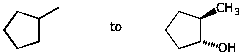
![]()