Organic Chemistry I |
Chapter 6 Notes |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Chapter 6 Notes |
Professor Carl C. Wamser |
![]()
![]()
Reaction Types
Alkene Addition Reactions
Addition Mechanism
Addition of HX to Alkenes
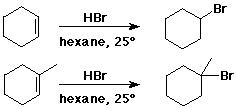
Orientation of Addition - Regiochemistry
Markovnikov's Rule
Markovnikov Addition
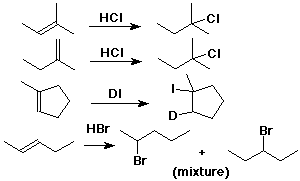
Hydration of Alkenes
Hydration Mechanism
Anti-Markovnikov Addition of HBr
follows a different mechanism involving free radicals
Br• adds first, forming the more stable (more substituted) radical
H is added second (from HBr) - to the more substituted C (anti-Markovnikov)
Catalytic Hydrogenation
CH2=CH2 + H2 ---> CH3-CH3
Stereochemistry - Syn Addition
1,2-dimethylcyclohexene + H2 --(cat)-->cis-1,2-dimethylcyclohexane (no trans)
Halogenation of Alkenes
CH2=CH2 + Cl2 ---> Cl-CH2-CH2-Cl
Stereochemistry - Anti Addition
cyclopentene + Br2 ---> trans-1,2-dibromocyclopentane (no cis)
Bromonium Ion
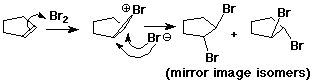
Halohydrins
Hydroboration/Oxidation
Oxidation of Alkenes
Epoxidation
addition of O to C=C, forms epoxide ring
reagent is peroxyacid ( RCO3H )
Oxidative Cleavage
C=C --> C=O + O=C
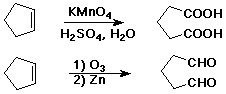
![]()