Organic Chemistry I |
Quiz 8 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Quiz 8 |
Professor Carl C. Wamser |
![]()
1. (6 points) Complete each of the following reactions by predicting the expected major product.
Show stereochemistry if it is specific. Also predict the most likely mechanism.
a) 
b) 
c) 
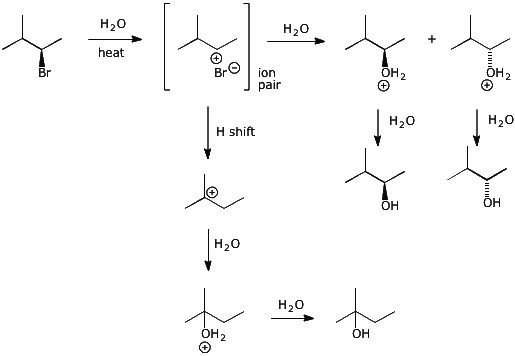
2. (4 points) Write a complete SN1 hydrolysis mechanism for the (R) stereoisomer shown below.
Show all steps including pathways to each of the main products:
2-methyl-2-butanol (50%), (S)-3-methyl-2-butanol (30%), (R)-3-methyl-2-butanol (20%)

![]()