Organic Chemistry I |
Final Exam |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Final Exam |
Professor Carl C. Wamser |
![]()
1. (25 points) Write complete names for each of the following.
a) 
b) 
c) 
d) 
e) 
2. (15 points) Write accurate structures for the following:
a) a Newman diagram showing the best conformation of 1-propanol
b) bicyclo[3.2.2]nonan-2-ol
c) a 3° alkyl bromide with molecular formula C6H13Br
d) a C5 compound in which all carbon atoms are sp2
e) the most stable chair conformation of (S,S)-1,2-dimethylcyclohexane
3. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) acidity
![]()
b) nucleophilicity
![]()
c) stability
![]()
d) ratio of substitution over elimination using KOH in EtOH
![]()
e) number of tertiary hydrogens
![]()
4. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific. Indicate whether the expected product would be optically active or racemic.
a) 
b) 
c) 
d) 
e) 
5. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 
b) 
c) 
d) 
e) 
6. (20 points) Write an accurate structure for (R,R)-3-chloro-4-methylhexane.
Then write a Newman diagram of that compound looking down the C3-C4 bond.
Show all three staggered conformations and select the one that will give the most stable E2 elimination product according to the Zaytzev Rule.
Show and describe the stereochemistry of the final alkene product (a complete structure and name).
7. (20 points) Show a sequence of reactions that could be used to synthesize 2-hexyne starting from propane as the only source of the carbons in your final product.
Hint - You should make propene and propyne as necessary ingredients for your synthesis.
Just show the necessary reagents and conditions and products for each separate reaction. Mechanisms are not required.
8. (15 points) Reaction of trans-2-bromocyclopentanol with HI gives primarily 1-bromo-2-iodocyclopentane.
Write an SN1 mechanism that shows this reaction with the expected products. Show all steps and show electron-pushing arrows.
Then write a modified mechanism to take into account the following stereochemical observations:
The product is entirely trans-1-bromo-2-iodocyclopentane; no cis is observed.
Furthermore, if the original compound was optically active, the product is racemic.
9. (10 points) Complete each of the structures on the right by adding the appropriate substituents so that they are identical to the structures on the left.
a) (3 pts) 
b) (5 pts) 
c) (2 pts) 
10. (14 points) Write a complete mechanism for the following dehydration reaction. Show all steps and electron-pushing arrows.

11. (20 points) Shown below are two possible reactions of chlorine with neopentane.
Calculate delta H for each reaction, using kcal/mol units.
a) ![]()
b) ![]()
Show reasonable mechanisms for each of the two possible reactions above. Just write a pair of propagation steps for each mechanism.
a)
b)
12. (16 points) Addition of BrCN to 1-methylcyclopentene could give different regioisomers and different stereoisomers depending on the mechanism. Assuming Br is the primary electrophile in this addition, illustrate the predicted products in each of the cases below.
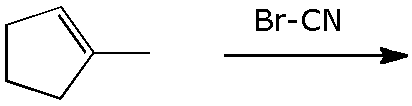
a) Markovnikov, syn stereochemistry
b) Markovnikov, anti stereochemistry
c) anti-Markovnikov, syn stereochemistry
d) anti-Markovnikov, anti stereochemistry
![]()