Organic Chemistry I |
Quiz 1 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Quiz 1 |
Professor Carl C. Wamser |
![]()
1. (4 points) The Lewis structures below are not the best forms. Write a better resonance form, specifically showing how you would push electrons (use curved arrows) to generate the better form. Explain briefly why one form is better than the other.
a) 
b) 
2. (2 points) Rewrite the line structure below to show all bonds and all lone pairs in approximately their correct geometry.
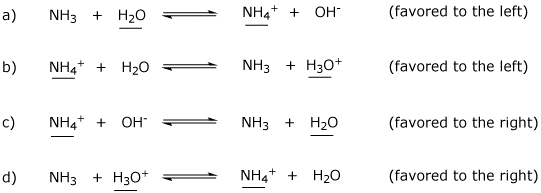
3. (4 points) Ammonia and water could conceivably undergo a variety of acid-base reactions. Complete each reaction, then identify the acid on each side. Indicate whether the reaction would be favored to the right or to the left.
Potentially useful pKa values are: H2O (15.7), H3O+ (-1.7), NH3 (36), NH4+ (9.3)
![]()