Organic Chemistry I |
Exam 1 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Exam 1 |
Professor Carl C. Wamser |
![]()
1. (16 points) Write complete names for each of the following. Include stereochemistry for the cyclic compounds.
a) ![]()
b) 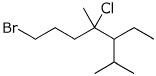
c) 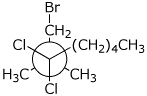
d) 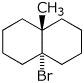
2. (15 points) Write accurate structures for the following:
a) gauche-butane, using wedge/dash to show the 3D structure (NOT a Newman diagram)
b) the best conformation of 1,1,3-trimethylcyclohexane
c) both resonance forms of NCO- (bonded in that order)
show all lone pairs and underline the better form
d) the conjugate base of acetic acid (CH3COOH )
show all lone pairs on both resonance forms
e) 1,5-dimethylspiro[2.3]hexane
3. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) acidity
H2S H2SO4 H2SO3
b) boiling point
c) heat of combustion (amount of heat evolved)
d) dipole moment
CH3Cl CH2Cl2 CCl4
e) stability
cis-1,2-dimethylcyclohexane cis-1,3-dimethylcyclohexane cis-1,4-dimethylcyclohexane
4. (9 points) Complete each of the following acid-base reactions. Identify the pKa values of the acids on each side of the equation and predict the preferred direction of the equilibrium.
a) ![]()
b) ![]()
c) ![]()
5. (16 points) Write line structures and names for all eight of the isomers of formula C5H11Br .
6. (13 points) Write Newman diagrams for all three staggered conformations of
1-bromo-2-methylbutane, looking down the C1-C2 bond.
Indicate the relative stabilities of the three forms.
7. (16 points) The chair conformation is shown below in both a line structure and a Newman diagram.
Carbons 1 and 3 are indicated to help you orient the views, which are intended to represent the same molecule.
Add the following substituents to both the line and Newman structures:
equatorial Br at C-1, axial OH at C-3, equatorial F at C-4, axial CH3 at C-6
Then write the other chair conformation in both views.
![]()