Organic Chemistry I |
Quiz 2 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Quiz 2 |
Professor Carl C. Wamser |
![]()
1. (4 points) Write complete names for the following compounds:
a) (CH3)3CCH2CBr2CH(CH3)2
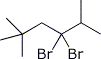 4,4-dibromo-2,2,5-trimethylhexane
4,4-dibromo-2,2,5-trimethylhexane b) 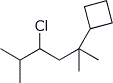
2. (3 points) Write an accurate structure for the following compound, clearly showing the 3-dimensional arrangement about every carbon. Show every bond and every lone pair and any formal charges
H2C=CH-CH2-Cl

For each sp3 carbon, indicate whether it is 1°, 2°, 3°, or 4°.
3. (3 points) Write a balanced reaction for the complete combustion of C3H6 .
Depending on the specific C3H6 compound, the heats of combustion are different. Explain what you can conclude about relative stability of these isomers.

![]()