Organic Chemistry I |
Quiz 7 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Quiz 7 |
Professor Carl C. Wamser |
![]()
1. (4 points) Write complete names for the following, including specific designation of stereochemistry.
a) 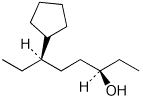
b) 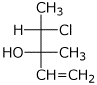
2. (2 points) If the pure (R) enantiomer of a compound has [a] = +15°, what would be expected as the observed optical rotation, a, for each case below (assuming a standard 1 dm cell is used)?
a) 0.5 g/mL of 50% (R) and 50% (S)
racemic, no optical activity, a = 0°
b) 1.0 g/mL of 80% (S) and 20% (R)
60% enantiomeric excess = 60% optical purity, a = - 9°
3. (4 points) Identify the relationship between the pairs of compounds
below.
Options are: E = enantiomers, D = diastereomers, C = constitutional isomers,
I = identical, F = conformational isomers, O = some other relationship
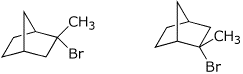 Enantiomers
Enantiomers Enantiomers
Enantiomers  Diastereomers
Diastereomers  Enantiomers
Enantiomers ![]()