|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Final Exam Answer Key |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Final Exam Answer Key |
![]()
1. (25 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 
(1S,2S,5R)-2-bromo-1,5-dimethylcyclohexanol
b) 
(S)-6,6-dichloro-3-methyl-3-cycloheptenol
c) 
(E,R)-7-methyldec-6-en-1-yn-5-ol
d) 
(2S,3S)-4-penten-1,2,3-triol
e) 
(2R,3S)-3-t-butyl-3-chloro-6-methyl-5-hepten-2-ol
2. (15 points) Write accurate structures for the following:
a) the conjugate base of 1-propyne
![]()
b) a compound of formula C3H4O with ONLY sp2 carbons
![]()
c) a good 3-dimensional structure for NH2OH (including lone pairs)

d) a Newman diagram of anti 1-propanol

e) a bicycloheptane of formula C7H12
 (any one)
(any one)
3. (15 points) Identify the relationships between the pairs of structures shown below using ONE of the following indications for each pair:
I - identical, C - constitutional isomers, D - diastereomers,
E - enantiomers, F - conformational isomers, O - other
a)  D
D
b)  C
C
c)  D
D
d)  I
I
e)  C
C
4. (15 points) Arrange the following in order with respect to the property
indicated.
Write MOST and LEAST under the compounds with the highest and
lowest values, respectively.
a) acidity
![]()
MOST / / LEAST / / MIDDLE
b) boiling point
![]()
MIDDLE / / LEAST / / MOST
c) nucleophilicity
![]()
MOST / / MIDDLE / / LEAST
d) rate of dehydration

MIDDLE / / LEAST / / MOST
e) heat of combustion

LEAST / / MIDDLE / / MOST
5. (15 points) Complete each of the following acid-base reactions and indicate whether the equilibrium is favored to the right or left.
a) ![]()
b) 
c) ![]()
d) 
e) ![]()
6. (15 points) Write Newman diagrams for all three staggered conformations
of
1-chloro-2-methylpropane, looking down the C1 - C2 bond.
Indicate which conformations could lead to E2 elimination in the presence of a strong base.
What would be the product(s) of E2 elimination?

7. (15 points) Initially using a planar structure for the ring, write the
structure of
(R,R)-1,3-dichlorocyclohexane.
Write both chair conformations of this compound.
Indicate which conformations could lead to E2 elimination in the presence of a strong base.
What would be the product(s) of E2 elimination?

8. (15 points) Calculate ΔH for the following reactions (using units of kcal/mol).
a) ![]()
bonds broken: 1° C-H = 98 and F-F = 38
bonds made: 1° C-F = 106 and H-F = 136
ΔH = + 98 + 38 - 106 - 136 = -106 kcal/mol
b) ![]()
bonds broken: CH3-CH3 = 88 and F-F = 38bonds made: two CH3-F = 108ΔH = + 88 + 38 - 108 - 108 = -90 kcal/mol
Can you tell from the ΔH values which reaction is more likely to
occur?
Explain why or why not.
NO.
Activation energies determine the likelihood of a reaction occurring.
9. (15 points) Write all the monochlorination products expected from the free radical chlorination of 2,2,4-trimethylpentane (names not needed, just structures).
Predict the relative amounts of each of the products, given that the selectivity of chlorination favors 3° > 2° > 1° by 5 : 4 : 1 .
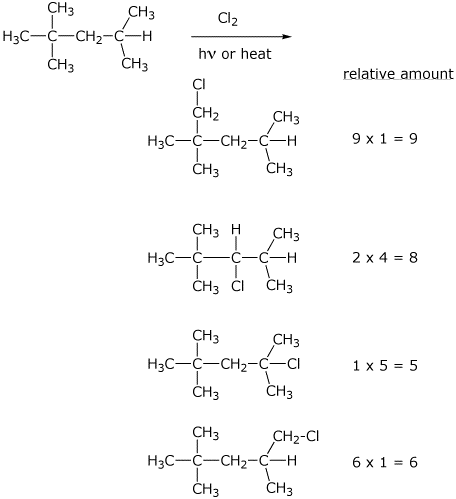
10. (20 points) Write a complete mechanism for the reaction shown below. Show all steps and electron-pushing arrows for each step. Follow the stereochemistry carefully, and indicate the expected stereochemistry at each stereocenter at every step of the reaction. Indicate whether the products would be expected to be racemic, achiral, or optically active.

11. (15 points) Cis-1-bromo-3-methylcyclopentane undergoes a slow SN1 and E1 process when heated in water. Write a complete mechanism, showing each step and electron-pushing arrows. Show the expected stereochemistry of the products.

Additional products observed include 1-methylcyclopentanol and 1-methylcyclopentene.
Expand your mechanism to show how these may be formed.

12. (20 points) Show how to synthesize the following compounds. You may use acetylene and methyl iodide as the only sources of carbon in creating the final products.
a) 

b) ![]()

![]()