|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 7 Notes |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 7 Notes |
![]()
![]()
Stereochemistry
Enantiomers
Enantiomer Examples
![]()


![]()
Chirality
Stereogenic Centers
Identifying Chiral Molecules


Optical Activity
Specific Rotation
Absolute Configuration
R and S Designations
Right- and Left-Hand Views
Drawing 3-D Structures

Fischer Projections
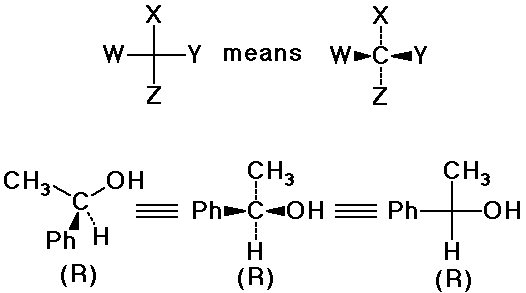
Multiple Stereogenic Centers
Diastereomers
Meso Compounds

Identifying Meso Compounds

Cyclohexane Derivatives
Configurations and Conformations of Disubstituted Cyclohexanes
|
|
|
|
|
|
(R,S) interconverting enantiomers |
(R,R) & (S,S) isolable enantiomers two conformations each |
|
|
isolable enantiomers two conformations each |
isolable enantiomers two conformations each |
|
|
(R,S) - meso compound two conformations |
isolable enantiomers two conformations each |
|
|
isolable enantiomers two conformations each |
isolable enantiomers two conformations each |
|
no stereocenters |
equivalent conformations |
two conformations |
|
no stereocenters |
two conformations |
two conformations |
Racemic Mixtures
Optical Purity / Enantiomeric Excess
Optical Resolution
Isomerism - Summary
Stereoisomers - Summary
Reactions involving stereoisomers
![]()