|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 2 Notes |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 2 Notes |
![]()
Molecular orbitals
Hybrid orbitals
Why hybrid orbitals?
Identifying the hybridization of carbon
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hydrocarbons
only C-H and C-C single bonds (alkanes)
C=C double bond (alkenes)
C=C triple bonds (alkynes)
rings (cycloalkanes, bicycloalkanes, polycycloalkanes)
aromatic rings (arenes)
Alkane Family
Higher Alkanes
Alkane Isomers
straight-chain
branched-chain
cyclic chain
atoms connected in a different order
butane and isobutane
3 pentane isomers
Butane isomers
n-butane 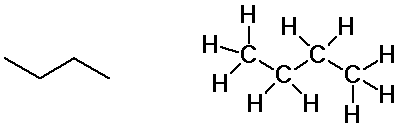
isobutane 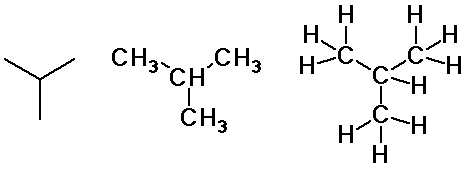
Pentane Isomers
Alkyl Groups
n-propyl alcohol ![]()
isopropyl alcohol ![]()
(constitutional isomers)
Butyl Groups
Classification of C Atoms
Identifying Carbon Classes
IUPAC Nomenclature
(substituents)-(parent alkane)-(family)
Cl-CH2CH2-OH
2-chloro ethan ol
IUPAC Rules
IUPAC Examples
IUPAC Examples
Cycloalkane nomenclature
Writing Skeletal Structures
Practice with Line Structures
![]() methylcyclohexane
methylcyclohexane
Alkanes - Source (Petroleum)
bp < 25° (C1-4) gas (burned off in flares)
bp 25-150° (C5-12) gasoline
bp 150-230° (C12-15) kerosene
bp 230-340° (C15-25) fuel oil
residue is asphalt
Alkanes - physical properties
nonpolar - low melting point, low boiling point, low water solubility
e.g., NaCl (ionic) bp 1413° , H2O (polar) bp 100° , CH4 (nonpolar) bp -161°
branched alkanes have lower bp than straight-chain analogs
( van der Waals forces depend on surface area)
Reactions of alkanes
Heats of combustion
Oxidation-reduction reactions