|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 3 Notes |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 3 Notes |
![]()
![]()
Conformations
Ethane Conformations
C-H bonds on the two carbons may or may not align
eclipsed: C-H bonds are aligned ![]()
staggered: C-H bonds fit in between 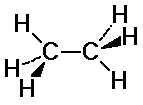
Newman Projections
Potential Energy Diagrams
Butane conformations
Types of strain:
Small cycloalkanes
Cyclohexane
Monosubstituted cyclohexanes
PRACTICE DRAWING ACCURATE CHAIR FORMS WITH SUBSTITUENTS
equatorial generally preferred over axial
see the table in the text with delta G for axial vs equatorial
Disubstituted cyclohexanes - cis & trans isomerism
Depending on the substitution pattern, disubstituted cyclohexanes have different combinations of axial and equatorial substituents.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Example - the two chair conformations for cis-1-chloro-3-methylcyclohexane involve either two axial substituents or two equatorial substituents. The latter is more stable.
![]()