Organic Chemistry |
||
Professor Carl C. Wamser |
||
Chem 334 - Fall 2003 |
EXAM 2 |
![]()
Organic Chemistry |
||
Professor Carl C. Wamser |
||
Chem 334 - Fall 2003 |
EXAM 2 |
![]()
1. (15 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 
b) 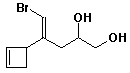
c) 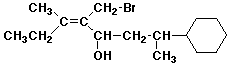
2. (15 points) Arrange each of the following in order with respect to the
property indicated.
Write “MOST” under the compound with the
highest value and “LEAST” under the compound with the lowest value.
a) stability
isobutyl cation < sec-butyl cation < tert-butyl cation
b) rate of dehydration
isobutyl alcohol < sec-butyl alcohol < tert-butyl alcohol
c) boiling point
isobutyl alcohol > isobutyl chloride> isobutane
d) rate of reaction with cyclohexene
F2 > Cl2 > Br2
e) rate of reaction with cyclohexane
F2 > Cl2 > Br2
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 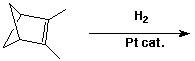
b) 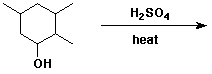
c) 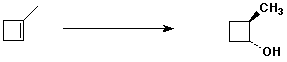
d) 
4. (10 points) A hypothetical reaction between chlorine and 2,2-dimethylpropane
is shown below.
Calculate ΔH for this reaction.
In fact, a different reaction takes place. Write it out as a balanced reaction and calculate ΔH.
5. (10 points) Strong acid can have the function of isomerizing alkenes, as shown below. Write a mechanism.
Another isomerization catalyzed by strong acid is shown below. Write a mechanism.
6. (15 points) Elimination from 1-iodo-4-methylcyclohexane follows the E2 mechanism when treated with KOH in ethanol. Write the conformation (chair) in which the cis isomer undergoes E2 elimination and illustrate the mechanism using electron-pushing arrows.
Predict whether the trans isomer would undergo E2 elimination faster or
slower than the cis isomer. Explain your reasoning.
7. (10 points) Write a complete mechanism for the radical chain polymerization
of propene.
Start with a general peroxide as initiator ( R-O-O-R ) and show
all initiation and at least two propagation steps.
8. (10 points) Photoinduced chlorination of 2,4-dimethylpentane can give
three different products having a single chloro substituent.
Write structures
for all three and predict the relative amounts of each, using a relative
reactivity of 3° > 2° > 1° hydrogens as 5 : 4 : 1 .
![]()