Alkanes and Cycloalkanes

Molecular orbitals
- overlap of atomic orbitals
- electrons are close to two nuclei
- bonding and antibonding combinations
Hybrid orbitals
- sp hybrids (one s plus one p)
- makes two identical orbitals (linear)
- sp2 hybrids (one s plus two p)
- makes three identical orbitals (trigonal)
- sp3 hybrids (one s plus three p)
- makes four identical orbitals (tetrahedral)
Why hybrid orbitals?
- good shape (directional)
- allows high overlap in bonding
- maximizes electron density between atoms
- good orientation
- minimizes repulsions between orbitals
Identifying the hybridization of carbon
- identify sigma and pi bonds around the carbon atom
(you need one sigma bond for each neighboring atom
and you need a total of four bonds for carbon)
|
Neighboring Atoms
|
Sigma Bonds
|
Pi Bonds
|
Hybrid
|
Structure
|
|
4
|
4
|
0
|
sp3
|
tetrahedral
|
|
3
|
3
|
1
|
sp2
|
trigonal planar
|
|
2
|
2
|
2
|
sp
|
linear
|
Hydrocarbons
- contain only C and H
- form basic carbon skeleton
- functional groups:
only C-H and C-C single bonds (alkanes)
C=C double bond (alkenes)
C=C triple bonds (alkynes)
rings (cycloalkanes, bicycloalkanes, polycycloalkanes)
aromatic rings (arenes)
Alkane Family
- methane CH4
- ethane CH3CH3
- propane CH3CH2CH3
- butane CH3CH2CH2CH3
- alkane CH3(CH2)nCH3
- CnH2n+2 (homologous series)
Higher Alkanes
- pentane C5H12
- hexane C6H14
- heptane C7H16
- octane C8H18
- nonane C9H20
- decane C10H22
- note the Greek prefixes
Alkane Isomers
straight-chain
branched-chain
cyclic chain
atoms connected in a different order
butane and isobutane
3 pentane isomers
Butane isomers
n-butane 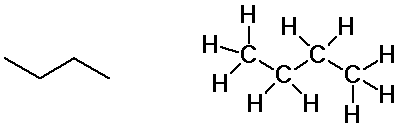
isobutane 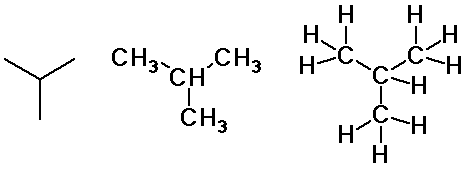
Pentane Isomers
- n-pentane
- isopentane
- neopentane
Alkyl Groups
n-propyl alcohol 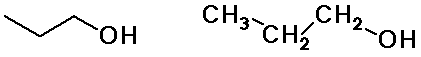
isopropyl alcohol 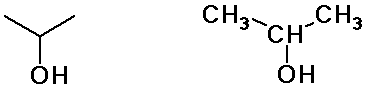
(constitutional isomers)
Butyl Groups
- n-butyl
- sec-butyl
- isobutyl
- tert-butyl
Classification of C Atoms
- 1° - primary - bonded to one other C
- 2° - secondary - bonded to 2 other C's
- 3° - tertiary - bonded to 3 other C's
- 4° - quaternary - bonded to 4 other C's
- class also applies to H atoms or functional groups attached to that
C
Identifying Carbon Classes
- identify the classes of each C, H and functional group in the molecule
below
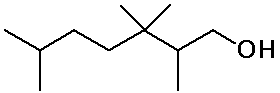
IUPAC Nomenclature
(substituents)-(parent alkane)-(family)
Cl-CH2CH2-OH
2-chloro ethan ol
IUPAC Rules
- parent = longest continuous carbon chain
- number chain from end closest to a substituent (1st difference)
- assign numbers to each substituent
- in naming, arrange substituents alphabetically
- for multiple substituents, use prefixes di-, tri-, tetra-, etc.
but they don't count when alphabetizing
- separate names from numbers with hyphens, numbers from numbers with
commas
- otherwise all written as one word
IUPAC Examples
- name the three isomers of pentane
- name the four isomers of C4H9Br
IUPAC Examples
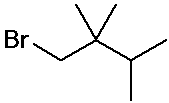
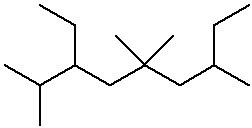
Cycloalkane nomenclature
Writing Skeletal Structures
- omit C-H bonds
- assume C makes 4 bonds
- omit C atoms
- assume C at end of every bond
- especially useful for cyclic structures
- be able to put back all the details
Practice with Line Structures
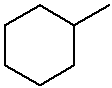 methylcyclohexane
methylcyclohexane
Alkanes - physical properties and sources
Reactions of alkanes
- combustion
- halogenation (later)
Heats of combustion
- comparison of relative stabilities of isomers
Oxidation-reduction reactions
- alkanes > alkenes > alkynes
- oxygen functional groups: alcohols > carbonyl (aldehyde/ketone) > carboxylic
acid > CO2
- other reactions
![]()
![]()

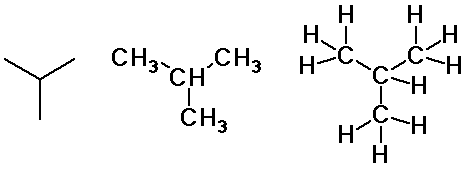
![]()
![]()
![]() methylcyclohexane
methylcyclohexane![]()