
Chem 334 - Fall 2001
Organic Chemistry I
Dr. Carl C. Wamser

1. (20 points) Write complete names for each of the following:
a) 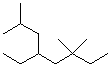
b) 
c) 
d) 
2. (10 points) Examine the structure below and answer the questions about it.
a) What is the molecular formula?
b) How many carbons of each possible hybridization are there?
sp3
sp2
sp
c) How many electrons are in pi bonds?
d) How many electrons are in lone pairs?
3. (15 points) Examine the pairs of structures below and identify their relationship to one another, using the letter codes below:
A - the identical structure
B - conformational isomers
C - resonance forms
D - constitutional isomers
E - cis-trans isomers
F - none of the above
a) 
b) ![]()
c) 
d) 
e) 
4. (15 points) Arrange the following sets of compounds in order with respect to the property indicated. Write "MOST" and "LEAST" under the compounds with the highest and lowest values of the property.
a) boiling point
b) boiling point
c) stability
d) stability
e) dipole moment
5. (15 points) Write both chair conformations for both cis and
trans isomers of 1,3-dimethylcyclohexane (label them A, B, C,
and D). Make your chair structures clear and accurate and identify
axial methyls by circling them.
cis-1,3-dimethylcyclohexane
trans-1,3-dimethylcyclohexane
Rank all four conformations in terms of their expected relative stability.
Most stable --------------------------- Least stable
6. (15 points) Write all three staggered conformations (label them A, B, and C) for
1,1-dibromo-2-methylpropane
(10 points) Also write an approximate potential energy diagram that illustrates the relative stability of each staggered structure, as well as the relative heights of the barriers (eclipsed conformations) between them. Label the axes of the energy diagram appropriately.