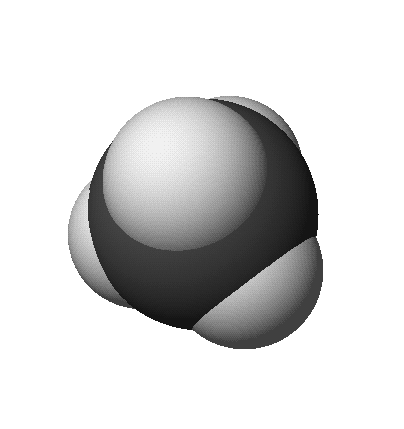Chapter 1 Notes: Overview / Bonding and Structure

Overview of Organic Chemistry
Organic chemistry
- carbon has unique chemistry
- bonds to every other element
- bonds to itself in long chains
- organic chemistry involves enormous variety
- many possible structures
- many possible reactions
Organic chemists
- what do organic chemists do?
- understanding structures, reactions
- correlation of structures with properties
- synthesis of compounds with specific properties
- who else needs organic?
- basis of all life processes
- the great variety of structures and reactions make life possible
How to handle variety
- nomenclature - clear methods for naming structures and reactions
- structures - organized by functional groups
- reactions - organized by reaction types (what happens?)
- reactions - organized by reaction mechanisms (how does it
happen?)
What should you get out of organic chemistry?
- from complex names, be able to derive a structure
- from complex structures, be able to identify functional groups,
predict characteristic properties
- work through reaction mechanisms - what is a molecule likely
to do under certain conditions
- "think like a molecule"
A reaction example
- CH3OH + HCl --> CH3Cl + H2O
- Reaction type
- Reaction mechanism
- how does the reaction occur ? (step-by-step)
Bonding and Structure
The Periodic Table
- atomic number (defines element)
- atomic weight (isotopes)
- electron shells (rows)
- groups (similar properties)
- filled shells (the noble gases)
- valence electrons (for bonding)
Bonding - Lewis structures
- the octet rule
- ionic bonding
- covalent bonding
- most common bonding in organic compounds
Typical valence - neutral atoms in normal bonding patterns
- H has 1 valence electron - makes 1 bond
- C has 4 valence electrons - makes 4 bonds
- N has 5 valence electrons - makes 3 bonds + 1 lone pair
- O has 6 valence electrons - makes 2 bonds + 2 lone pairs
- F has 7 valence electrons - makes 1 bond + 3 lone pairs
- Recognize the appearance of these common atoms in correct
structures
- Also recognize the common charged forms:
|
Atom |
Bonds |
Lone
Pairs |
Charge |
|
H |
1 |
0 |
0 |
|
H+ |
0 |
0 |
+1 |
|
H- |
0 |
1 |
-1 |
|
C |
4 |
0 |
0 |
|
C+ |
3 |
0 |
+1 |
|
C. |
3 |
1 e- |
0 |
|
C- |
3 |
1 |
-1 |
|
N |
3 |
1 |
0 |
|
N+ |
4 |
0 |
+1 |
|
N- |
2 |
2 |
-1 |
|
O |
2 |
2 |
0 |
|
O- |
1 |
3 |
-1 |
|
O+ |
3 |
1 |
+1 |
|
F |
1 |
3 |
0 |
|
F- |
0 |
4 |
-1 |
Writing organic structures
- Lewis structures
- all electrons shown
- Kekule structures
- show bonds as lines
- lone pairs sometimes omitted
- condensed structures
- common groups are abbreviated (e.g., CH3CH2 )
- line structures
- omit lone pairs
- omit hydrogens on carbons
- omit carbons
(assumed to be at the end of every bond)
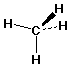
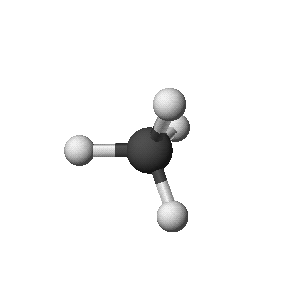
3-Dimensional structures
- dotted-line / wedge
- ball-and-stick
- space-filling
Visualizing chemical structures
- Name (common or systematic)
- Condensed Formula (as usually typed out)
- Lewis Structure (all atoms and bonds shown)
- Line Structure (omit hydrogens, assume carbons at vertices)
- 3-D Structure (show bond orientations)
- Ball-and-Stick Structure (like a molecular model you could
make)
- Space-Filling Model (approximates full size of electron distribution)
methane: CH4 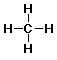
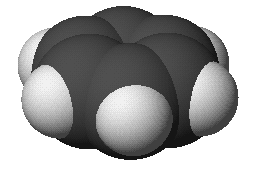
benzene:
C6H6 
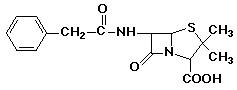
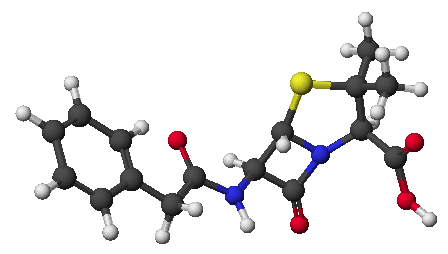
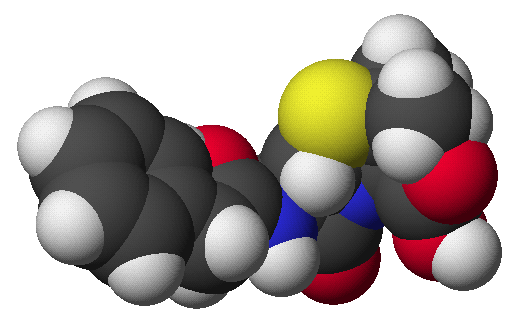
penicillin:
Try
a 3-D image:
1) Click at the bottom after you read these instructions
2) Cross your eyes to see two double images
3) Make the middle images merge
Try it!
Atomic orbitals
- wavefunctions
- describe location of electrons
- s orbital (spherical)
- p orbitals (three: x,y,z)
- (dumbbell shape - 2 lobes)
- d orbitals (4 lobes)
- not usually needed for organic chemistry
- hybrid orbitals
- combination orbitals
Bonding
- attraction between negative electrons and positive nuclei
- repulsions between electrons
- repulsions between nuclei
- bonding is a balance between the attractions and repulsions
- characteristic bond lengths and strengths
Molecular orbitals
- overlap of atomic orbitals
- electrons are close to two nuclei
- bonding and antibonding combinations
Hybrid orbitals
- sp hybrids (one s plus one p)
- makes two identical orbitals (linear)
- sp2 hybrids (one s plus two p)
- makes three identical orbitals (trigonal)
- sp3 hybrids (one s plus three p)
- makes four identical orbitals (tetrahedral)
Why hybrid orbitals?
- good shape (directional)
- allows high overlap in bonding
- maximizes electron density between atoms
- good orientation
- minimizes repulsions between orbitals
Identifying the hybridization of carbon
- identify sigma and pi bonds around the carbon atom
(you need one sigma bond for each neighboring atom
and you need a total of four bonds for carbon)
|
Neighboring Atoms |
Sigma Bonds |
Pi Bonds |
Hybrid |
Structure |
|
4 |
4 |
0 |
sp3 |
tetrahedral |
|
3 |
3 |
1 |
sp2 |
trigonal planar |
|
2 |
2 |
2 |
sp |
linear |
Molecular geometry by VSEPR
- electron pairs repel one another, maximize their separation
Electronegativity
- tendency of an atom to attract electrons in a covalent bond
- in the Periodic Table, electronegativity increases to the
right and up
- F > O > Cl ~ N > Br > C > H > metals
Polar covalent bonds
- electrons in a covalent bond may not be equally shared
H-Cl is polarized with excess electron density closer to Cl
Polar bonds to carbon
- C-C bonds are nonpolar
- C-H bonds are generally considered nonpolar
- C-X bonds are polarized with carbon partially +
for X = F, Cl, Br, I, O, S, N
- C-M bonds are polarized with carbon partially -
for M = metals
Functional Groups
- a specific arrangement of atoms
- define chemical families
- determine chemical properties
- basis of nomenclature
- organization of textbooks
- examples - the simple oxygen families:
- alcohols, ethers, aldehydes, ketones, carboxylic acids
Resonance
- more than one possible Lewis structure for a compound
- What's the best Lewis structure?
- follow the octet rule
- electronegativity determines the best place to locate charges
carbon monoxide (CO)
nitromethane (CH3NO2)
Electron-pushing
- keeping track of electrons is crucial in organic chemistry
- curved electron arrows indicate electron pair movement
- in converting resonance forms, or later, in reactions