closed system
energy in each component goes until they are equal
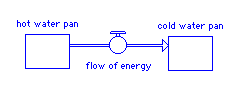
model of equilibrium
closed system
energy in each component goes until they are equal
Simple heat flow model
heat reservoir - stock - pan of water with 1 kg of water
heat input - Kcal per minute in
heat output - Kcal per minute out
control of heat in
control of heat lost
temperature of water
temperature of air
flux of heat depends on difference in temperature
heat_out = constant*flux_constant*(pan_temp - air_temp)
example output - describe why it looks this way
run this simulation at different values for burner_control and heat_flux_constant
general description